Squamous Cell Carcinoma of the Breast: A Case Report and Review of Literature
Introduction
Globally, breast cancer is the most common malignancy seen in
women, responsible for 25% of all cancers seen [1]. It is surpassed
by lung cancer alone in terms of incidence and cancer related
mortality and it was reported to be responsible for 6.6% of all
cancer related death in women in 2018 [1]. The overall incidence
and cancer related mortality is worse in developing countries.
The yearly incidence in West Africa is estimated to be about 33%
of all new cancer cases and it is said to be responsible for 24%
of cancer deaths in 2018 [2]. There is no significant difference in
the overall incidence and breast cancer related fatality rate in the
different parts of Nigeria. In Ibadan, south-western Nigeria, it is
the most prevalent female malignancy; accounting for 40.8% of
all cancers seen at the University College Hospital [3,4]. Reports
from Jos, North-central of Nigeria and Kano, North-western Nigeria
placed breast cancer as the most common malignancy in women
[5,6]. A report from Enugu, South-Eastern Nigeria also indicated an
incidence of 16.9% and reports from North-Eastern Nigeria is of the
same trend [7,8]. The most consistent factor in all the reporting is
the noticeable rising trend in Nigeria. This has been attributed to an
actual increase in incidence, increased awareness of breast cancer
in the populace, and an improvement in access to health care and
diagnostic tools [9,10]. Also, an upward trend in more aggressive
histological types, younger age of occurrence at presentation and
the presence of more biologically aggressive histological subtypes
and triple negative molecular profile are said to be on the rise in
Nigeria [10].
All over the world the most reported histological subtype is Invasive Ductal Carcinoma, constituting about 60-80%, although; a rare report has placed it to be about 50% [11]. This is similar to reports from Ibadan, south-west of Nigeria. The main histological subtype reported is invasive ductal carcinoma responsible for 89.2% of the cases [11]. This is the consistent finding in the south western part of the country [12,13]. The North-Eastern part of Nigeria where this report is coming from also has similar reporting [11,14]. The special types of breast cancer are said to occur at a very infrequent rate than that of the invasive ductal carcinoma. These include invasive lobular carcinoma, mucinous carcinoma, apocrine carcinoma, tubular carcinoma, papillary carcinoma, squamous cell carcinoma and lymphoma. The reported incidence from North- Central of Nigeria ranged from 3.9% to 5% [15]. The relative rarity of these subtypes is also reported in the South-Eastern [16], North- Eastern and South-Western parts of Nigeria [17,18]. Other parts of West Africa and the rest of the world also reported same pattern [19,20].
The squamous Cell Carcinoma of the breast is reported to be less than 0.1% of all the breast malignancies [21-23]. Its clinical presentation and radiological appearance are said to be unremarkable [23,24,26]. The complete metaplasia of ductal epithelium is the most widely reported mode of pathogenesis. [22,25,27]. It may arise as a sequelae of squamous metaplasia from chronic irritation, as seen in a chronic breast abscess and in the presence of non-biological breast implants [4]. It may arise from squamous metaplasia of a pre-existing adenocarcinoma of the breast [23,24,26,28]. It has been reported to arise from squamous metaplasia in benign lesions such as Epidermoid Cyst and Fibroadenoma [29,30]. It is important to differentiate from histopathological assessment, a primary Squamous Cell Carcinoma (SCC) of the breast from secondaries, either from direct extension from overlying skin or from extra mammary origin. This can be achieved through the exclusion of SCC of the overlying skin of the breast or nipple, secondaries from extra mammary origin, presence of unequivocal dominance (more than 90%) of areas with SCC, and the lack of other neoplastic cells of ductal or mesenchymal origin [31]. Immunohistochemical study of the cytokeratin profile of any tumour helps in making this distinction. The presence of diffuse CK7, CK8, CK19 staining and focal Carcino-Embryonic-Antigen positivity favors ductal origin [32-35]. The presence of membranous E-cadherin staining is diagnostic of ductal origin also [36,37]. A positive staining for HMW CK, histologic appearance and lamellar keratin formation are typical of squamous cell origin [36,37].
We present a case of a 43-year old married woman on long term steroid therapy, who developed malignant features in an 11-month old left breast lump 2 months prior to presentation. An initial diagnostic breast USS and Fine Needle Aspiration study were equivocal. A wide local excision was done and the postoperative pathological analysis revealed a primary SCC of the breast. We report the case because of its rarity and for being the first reported case from North-Eastern Nigeria.
Case Report
A 43-year-old Kanuri woman who resides in Maiduguri-Nigeria. She noticed a painless lump in the retroareolar region of the left breast 11-months ago. It was initially the size of a peanut, grew slowly over 9 months and started growing rapidly in the last two months. It developed a continuous dull aching pain, intermittently relieved with non-opiate analgesics and is currently the size of her fist. There is no ulceration, nodules or orange peel appearance of the overlying skin and the nipple-areolar complex is said to be preserved. There was no preceding history of nipple eczematous rashes, bloody discharge, deviation or retraction. A month prior to presentation, she noticed a painless left axillary mass with no ulceration of the overlying skin or ipsilateral arm swelling. The contralateral breast, axilla, neck and arm are said to be free of masses or swelling. She noticed progressive weight loss, anorexia and easy fatigue 2 months before presentation. There was no history suggestive of visceral, brain or bony metastasis. She attained menarche at the age of 14 years, married at 18 years and had her first child at 30 years. She gave birth to 7 children, breast fed all for an average of 18 months and has never used contraceptive drugs. There is no history of a first or second degree relative with breast malignancy. She was diagnosed with Scleroderma 12 years ago, has been on intermittent steroid, immunosuppressive and cytotoxic drugs for 5 years now.
An anxious young woman was seen with no evidence of physiologic derangement on general physical examination. A 10*10 cm mass was found in the retroareolar area, non-tender, hard, irregular with well-defined margins. The overlying skin showed peau d’orange and a solitary malignant nodule. There is an obvious nipple retraction and a bloody discharge was notice on expressing all the quadrants of the breast. There is a solitary, 2*2 cm, discreet, anterior axillary lymph node. It was not warm or tender, hard and irregular with free intrinsic mobility. The contralateral infra and supraclavicular spaces, contralateral breast and axilla were free. No clinical evidence of distant metastasis was noticed. A provisional clinical diagnosis of left breast mitotic lesion (T3N1Mx) to rule out chronic breast abscess was made.
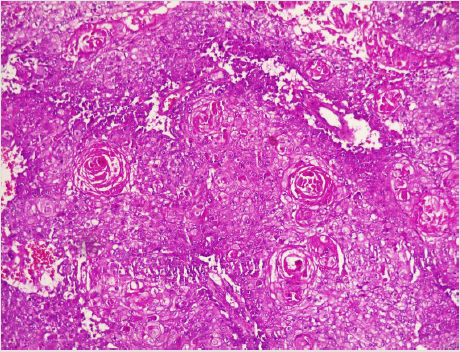
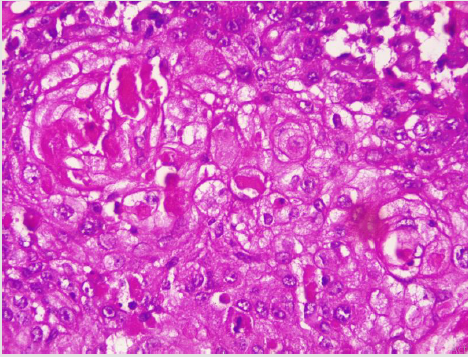
A diagnostic breast USS with a 5 MHz probe was done. A thickwalled 12 cm cystic lesion with multiple septations was seen. It has a 3 cm solid component, with no calcification or increased flow on Doppler interrogation. A fine needle aspiration was done, about 60 mls of straw-coloured fluid was drained and cytological analysis of the aspirate showed suspicious ductal cells. A wide local excision with axillary dissection and immediate breast reconstruction was done. Histopathological analysis showed malignant squamous epithelial cells that are disposed in sheets and nests with focal areas of keratin pearls (Figure 1A). The cells are polygonal with intercellular bridges (Figure 2B). Immunohistochemical staining with HMW Cytokeratin Antibody was positive. Immunohistochemical staining for focal Carcino-embryonic-antigen and E-cadherin was negative. The resection and deep margins are free of tumour and only the enlarged lymph node showed metastatic deposit. No adjuvant chemotherapy or radiotherapy given. Immunohistochemical study of molecular profile showed positivity for oestrogen only, progesterone receptor was negative and there is no over expression of the Her2-Neu receptor. Patient is currently on adjuvant selective oestrogen receptor modulator (Tamoxifen) at 20 mg daily for a two and a half years and will be subsequently switched to an Aromatase inhibitor {Anastrazole) for the same duration. Patient is to continue with follow-up visits to the surgical-out-patient-department. An informed consent was obtained from the patient for publication of the case report.
Figure 1: Photomicrographs of malignant squamous epithelial neoplasm that are disposed in sheets with focal areas of keratin pearls (A), A=X100.
Discussion
There is no consensus on the origin of squamous cell carcinoma, whether it is a pure form of squamous cell carcinoma or it represents an extreme squamous metaplasia of an existing adenocarcinoma [39]. It is thus classified in to primary pure SCC, like our reported case and a mixed type (Figures 1 and 2). The primary pure SCC is characterised by the following features: The predominance (>90%) of malignant cells being of the squamous cell type, having no direct relation with the overlying skin of the breast, nipple and the areola, and the absence of evidence for a primary in an extra mammary site [40,41]. The mixed type is seen following a squamous metaplasia of an existing adenocarcinoma or a metastasis from an extra mammary origin [42]. The primary Squamous cell metaplasia is known to occur in benign epidermoid, dermoid and sebaceous cysts of the breast [43], as seen in our patient. It may arise within a long standing Fibroadenoma [44]. Primary squamous cell carcinoma of the breast has been observed to arise from squamous metaplasia of ductal epithelium in benign disorders of the breast, such as chronic abscess, after implantation of breast prosthesis and after radiation therapy [45-49]. Although there was USS evidence of a cystic mass in our patient, there was no history suggestive of chronic inflammation, use of implant for breast reconstruction or administration of therapeutic ionizing radiation.
The reported age of high incidence was the 5th -6th decades of life, with an average age of 50 years [50]. Our patient presented at the age of 43 years, almost a decade younger than the age in developed countries. The average age at presentation is 37.3 years in Enugu, south-eastern Nigeria [50]. Like the frequently seen adenocarcinoma, squamous cell carcinoma of the breast is also predominantly seen in post-menopausal women worldwide [4], but, some pockets of studies have reported seeing SCC in women within their gestational or lactating periods [23,32,51]. The decade age gap in the age of occurrence of SCC between women of Africa and those in Europe and North America has been observed by several authors in Nigeria and the sub Saharan Africa. The mean age of the patients at Calabar, South-South of Nigeria was 45.06 years, with an age range of 23 to 76 years [52]. This is similar to reports of the common age of 31-40 years in Lagos, South Western Nigeria [53], 40-49 years in Maiduguri, North Eastern Nigeria [54], and 40-49 years in Ghana, West Africa [55]. The reported ages in developed countries are 40-50 years in Hong Kong, 75-85 years in Maryland-USA and a median age of 62 years for all the histological subtypes [56].
Majority of patients with SCC present with a breast lump, usually a solid mass [25,57], but a significant proportion present with a fluctuant cystic mass, mostly a malignant epidermoid or sebaceous cyst or even a chronic breast abscess with a solid malignant component [58-61]. Our patient presented with a complex mass consisting of both solid and cystic components, probably a benign breast cyst with subsequent squamous metaplasia. Majority of SCC of the breast are said to be biologically aggressive, characterized by rapid growth over short duration and subsequent cystic and necrotic degeneration [28,60,61]. The index patient noticed such rapid growth with associated nodule formation just 2 months prior to presentation. Like mucinous adenocarcinoma, Squamous cell carcinoma of the breast is reported to be bulky, most often measuring greater than 5 cm (T3) in its widest dimension [59,60,61]. Our index mass measured 10 cm in its widest diameter. Many reports have indicated the preponderance of the left breast as the site of squamous carcinoma more than the right [43]. Our patient also presented with a left breast mass. Wynder et al. have reported the tendency for left-sided preponderance of the carcinoma of the breast of all types.
No identifiable risk factors were noted in the index patient, except for the age, presence of a breast cystic mass and long term steroid therapy. It has been noted above that most SCC in the African women are seen in the 4th decade of life [52-55], just like the age of 43 years at presentation for our patient. The presence of a cystic mass also reinforced the suspicion of a squamous metaplasia of the epithelium on the cyst wall, as reported by many authors [58,59]. The use of steroid and immunosuppressive drugs for more than 10 years could have impaired the body’s ability for immunologic surveillance, to arrest progression from dysplastic state and to halt lymphatic metastasis. Our patient presented with a clinically palpable ipsilateral axillary lymph node that showed positive metastasis on histology. Surprisingly, most reports indicated that Squamous cell carcinomas do not readily metastasize through the lymphatics like the adenocarcinomas. In fact, in only 10-30% of cases is lymph node involvement encountered intra-operatively [26,42,45]. However, up to 30% of the patients may harbour distant metastases at the time of presentation [26,42].
A diagnostic breast USS with a 5 MHz probe was done. A thickwalled 12 cm cystic lesion with multiple septations was seen. It has a 3 cm solid component, with no calcification or increased flow on Doppler interrogation. Other authors have reported also that there are no typical findings on the mammogram and a breast USS may only reveal a complex cystic mass with solid component or an inflammatory process [42]. Calcification and Cystic changes are often the frequent radiological finding [24-26,58]. A fine needle aspiration was done, about 60 mls of straw-coloured fluid was drained and cytological analysis of the aspirate showed suspicious ductal cells. A Tru-cut biopsy is the preferred method of obtaining a pathological diagnosis, as fine needle aspiration study is often not helpful [42]. In a study done by Gupta RK et al, very few patients have the histological diagnosis confirmed via a fine needle aspiration [62,63]. A wide local excision with axillary dissection and immediate breast reconstruction was done. Histopathological analysis showed malignant squamous epithelial neoplasm that are disposed in sheets and nests with focal areas of keratin pearls (Figure 1A). The cells are polygonal with intercellular bridges (Figure 2B). The reported histological evidence of a primary SCC of the breast is the microscopic appearance of malignant cells composed of infiltrative nests of atypical epithelial cells with irregular and hyperchromatic nuclei. There may be variable amounts of keratinous lamellar or a pseudo-sarcomatoid stroma [64-68]. It may have associated Ductal Carcinoma In-Situ or squamous metaplasia of ductal epithelium at the periphery of the invasive carcinoma [23,25,26,59,69]. Immunohistochemical staining with HMW Cytokeratin was positive. immunohistochemical staining for focal Carcinoembryonic- antigen and E-cadherin was negative. For adequate treatment, it is imperative to separate cutaneous SCC from primary SCC of the breast. Apart from the requirement of the predominance (>90%) of the squamous malignant cells in all the arrears of the tumour, the tumour should have no continuity with overlying epidermis or dermal appendages [31]. Immunohistochemical staining has emerged as an important diagnostic tool in making such distinction. Both the pure primary SCC of the breast and the SCC extending in to breast parenchyma from overlying skin stain positive with HMW-CK antibody. But, only primary pure SCC shows diffuse positive staining for CK 7, CK 8 and CK19 Antibodies [33- 36]. The distinction of pure primary SCC from secondary SCC from extra mammary sites such as the lungs is also difficult, because SCC differentiation demonstrate identical immunohistochemical staining in every organ in the body [21]. The demonstration of MAP2K4 gene mutation in a biopsied specimen should confirm an extra mammary origin, especially a lung SCC [21,70].
The resection and deep margins are free of tumour and only the enlarged lymph node showed metastatic deposit. No adjuvant chemotherapy or radiotherapy given. Literature review has shown that surgery is the mainstay of the treatment and a definitive role for adjuvant therapy has not been determined [24-26, 57- 59]. Both adjuvant radiotherapy and chemotherapy have been used with variable outcomes [24,26,47,57]. Majority of reports did not demonstrate a superior oncologic outcome with the use of adjuvant radiotherapy in primary SCC of the breast in stark contrast to extra mammary primary SCC [28,71]. Some studies have demonstrated an objective response and subsequent down staging of locally advanced pure primary SCC of the breast with the use of combined Neoadjuvant chemotherapy and radiotherapy [57,58]. Immunohistochemical study for molecular profile showed positivity for oestrogen only and progesterone receptor was negative, with no over expression of the Her2-Neu receptor. Patient is currently on adjuvant selective oestrogen receptor modulator (Tamoxifen) at 20 mg daily for two and a half years and will be subsequently switched to an Aromatase inhibitor for the same duration. The pure primary Squamous cell carcinomas are known to be mostly hormone receptor negative [40,41,46]. The over expression of c-erbB-2 is also usually not seen, but, some few reports have indicated an overexpression [69,72]. Patient is to continue with follow-up visits to the surgical-out-patient-department.
Because of its rarity, no much data has been published concerning the disease free survival and overall survival after curative treatment. A single-centre, small sample-size, retrospective study reported a 5-year survival of 67% in [26]. Menville has reported that prognosis is dependent upon the tumour biology and grade and may vary between deaths within 4 months in metastatic disease, to 16 years in early disease with curative resection [29]. Although the clinical course and prognosis of the SCC of the breast have remained obscure, many reports have considered a young age below 40 years, large tumour size and a histologically proven nodal metastasis to be markers of poor prognosis [25,26,57,71].
Conclusion
Despite its rarity, a primary squamous cell carcinoma of the
breast is of clinical significance, because it can masquerade as
a benign mass or arise within an existing adenocarcinoma. It’s
often high histological grade, lack of hormone receptor positivity
and often poor response to adjuvant care make its treatment
herculean. Every benign breast mass removed must be subjected to
pathological analysis, especially chronic cysts and abscesses.
For more Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.