Hylan A: A Novel Transporter for Latanoprost in the Treatment of Ocular Hypertension
Introduction
Glaucoma is the second leading cause of blindness after
cataracts and is usually irreversible [1]. It is expected that by
2020 approximately 76 million people suffered from glaucoma
and that this number will reach 112 million by 2040 [1]. Ocular
hypertension (OHT) is a leading risk factor for the development
of POAG and, lowering the intraocular pressure (IOP) is the only
therapeutic option to prevent or delay the development of POAG
[2]. Prostaglandin analogues such as latanoprost are the most
effective active pharmaceutical ingredients (API) for lowering the
IOP [3]. Current eye drops for the treatment of OHT and POAG
are known for their high incidence of immediate as well as longterm
undesirable side effects [4]. They are associated with a high
incidence of progressive glaucoma therapy-related ocular surface
disease (OSD) [5]. Undesirable side effects may either be intrinsic,
i.e. caused by the API or caused by excipients contained in the
vehicle [6]. The vehicle is intended to dissolve or suspend the API,
stabilize the solution during shelf-life of the eye drops and during
patient use, prolong the contact time between the API and the
ocular surface, support the penetration of the API into the ocular
surface, and enhance the biocompatibility of the eye drops [7,8].
Latanoprost in aqueous solution is known to require stabilization
[9-13]. Today most commercial latanoprost eye drops contain the
quaternary ammonium cationic detergent benzalkonium chloride
(BAC) as a preservative and penetration enhancer. The devastating
long-term effects of BAC on the ocular surface are proverbial [14].
Recently preservative-free IOP lowering eye drops have become
available, avoiding the long-term effects of BAC [5,15,16]. Although
less oculotoxic, these eye drops also contain penetration enhancers
such as EDTA, compromising the epithelial barrier, with the potential
of causing long-term damage to the ocular surface [17-19]. The
use of hyaluronic acid (HA) as mucoadhesive thickening agent in
drug vehicles has been suggested in the literature [20-28]. HA has been
shown to counteract the irritating effect of substances to the
ocular epithelium [29-33]. Recent studies have shown that hylan A
in eye drops does not only provide hydration and lubrication of the
ocular surface, but seems to enter the epithelium, where it acts
antiinflammatory
and provides neurotrophic support to compromised
epithelial nerves [34-36]. The current study intended to investigate,
if the polyanion hylan A is able to act as an influx transporter
enhancing the solubility and stability of latanoprost and facilitating
its translocation across the ocular epithelial barrier [37].
Materials and Methods
Materials
Sterile bulk solution for the production of Comfort Shield® MDS eye drops (i.com medical GmbH, Munich, Germany) was used as vehicle for the preparation of prototype latanoprost bulk solution (PLBS). The vehicle contained 0.15% w/v hylan A (HA with 2.9 m³/kg intrinsic viscosity) dissolved in phosphate buffered saline solution (8.035 g/l NaCl; 1.2 mmol/l Na2HPO4/NaH2PO4; pH 7.4). Latanoprost was obtained from Yonsung Fine Chemicals Co. Ltd., Gyeonggi-do, Republic of Korea. PLBS was prepared by medipharm Laboratorium GmbH, Falkensee, Germany by dissolving 20+1 μg/ml latanoprost in the vehicle. Sterile 10 ml bottles with Ophthalmic Squeeze Dispenser (OSD) were obtained from Aptar Radolfzell GmbH, Radolfzell, Germany. Medi-pharm Laboratorium GmbH prepared two batches of prototype latanoprost test samples (PLTS-A) for stability screening, by aseptically filling 9 ml of PLBS into Aptar bottles and closing them with the OSD dispenser.
Sterile Novelia® 11 ml soft bottles and PureFlow® 1500 droppers with valve diameter 1.6 were obtained from Nemera, La Verpillière, France. Pharmpur GmbH, Königsbrunn, Germany prepared prototype latanoprost test samples (PLTS-N) for the IOP self-test by aseptically filling 10 ml of PLBS into sterile Novelia bottles and closing them with the droppers. These test samples had to be kept vertical to minimize the contact between the solution and the dropper, as latanoprost has a tendency to adsorb to silicone parts contained in the dropper. The latanoprost concentration in the PLTS-N bottle used for the self-test was 19 μg/ml. Xalatan® eye drops (PFIZER OFG Germany GmbH, Berlin, Germany) containing 50 μg/ml latanoprost, 0.2 mg/ml BAC and 6.3 mg/ml phosphates were used as comparative samples in the IOP self-test. Comfort Shield® MDS 0.15% hylan A eye drops (i.com medical GmbH, Munich, Germany), composed of the vehicle of the prototype latanoprost test samples, were used as control and during the wash-out period in the IOP self-test.
Test Subject
The test subject (TS) is a 71-year-old, male, with healthy ocular surface, without history of ocular trauma or ocular surgery or use of preserved eye drops, with untreated ocular hypertension not associated with glaucoma.
Methods
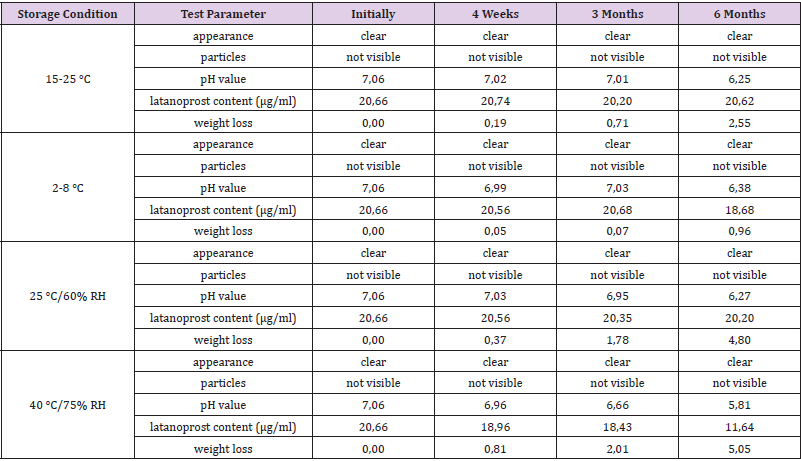
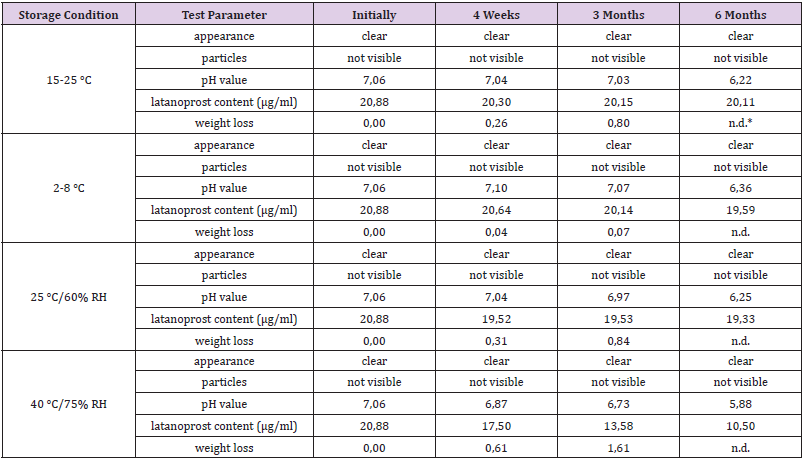
In a pre-test 25 μg/ml and 50 μg/ml latanoprost were added to the vehicle and the solution stirred for 18 hours at 40 °C. The latanoprost content was determined by HPLC using a Hypersil BDS C18 5μg, 150.0x4.0 mm column (VDS optilab) and a UV detector (200 nm). Independent from the starting amount, 20.8 μg/ml latanoprost were found to be dissolved in the vehicle as compared to a solubility of 12.9 μg/ml latanoprost in water [38]. Samples from two batches of PLTS-A were stored for six months at ambient room temperature (15-25 °C), at 2-8 °C, at 25 °C / 60% RH, and at 40 °C / 75% RH. Initially, after 4 weeks, after 3 months, and after 6 months samples were visually inspected for appearance (clarity) and absence of particles, and tested for pH value, latanoprost content, and loss of weight. Throughout the self-test IOP was measured using a hand-held iCare HOME Model TA022 rebound tonometer intended for self-use (Icare Finland Oy, Vantaa, Finland) [39]. Triple measurements were taken, and the average values noted.
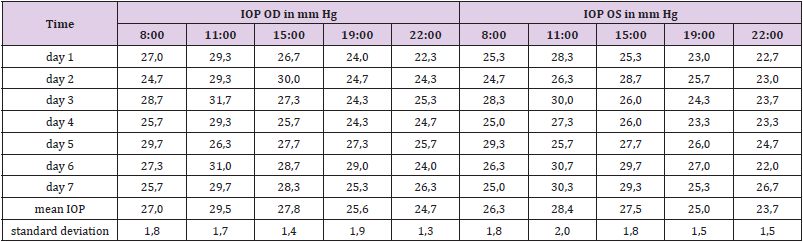
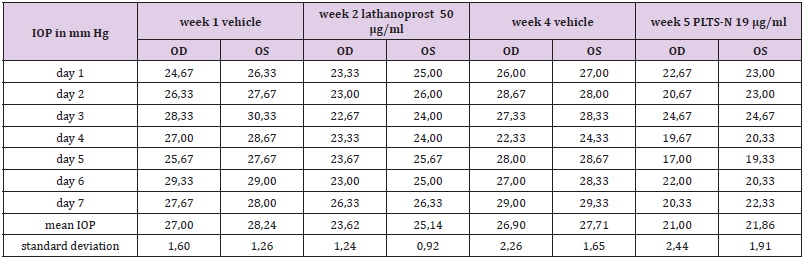
IOP is known to fluctuate widely during the 24-hour (circadian) period, and, moreover, that the time of the peak of IOP varies from patient to patient [40, 41]. Less is known about the day to day (interdian) variability of the IOP. Therefore, the TS measured the IOP of both eyes on seven consecutive days at 08:00 (8 a.m.), 11:00 (11 a.m.), 15:00 (3 p.m.), 19:00 (7 p.m.) and 22:00 (10 p.m.). The individual time of peak IOP of the TS (11:00) was chosen for the IOP monitoring throughout the screening test. Eight weeks before the self-test the TS applied one drop of Comfort Shield MDS eye drops (= vehicle) in each eye in the morning and evening. The self-test lasted over a period of five weeks, and the IOP of both eyes was measured daily at 11:00. In week 1, 3, and 4 the vehicle was applied in the morning and evening. During weeks 2 and 5 the vehicle was only applied in the morning (between 7:00 and 8:00) and one drop of latanoprost eye drops was instilled in each eye between 19:00 and 20:00 in the evening. During week 2 Xalatan® eye drops (50 μg/ml latanoprost) were applied, whereas, in week 5 PLTS-N eye drops (19 μg/ml latanoprost) were instilled.
Results
Stability Screening
The results of stability screening for two batches of prototype latanoprost test samples (PLTS-A) are summarized in Tables 1 and 2.
IOP Self-Test
In order to study the individual circadian rhythm and interdian variability the TS performed IOP measurements on seven consecutive days. The IOP of the TS reached a peak in the late morning followed by a continuous decrease until the evening (see Table 3). Based on the results of this test it was decided to perform IOP measurements at 11:00 for the comparison of the effectiveness of latanoprost eye drops in the eyes of the TS. There were also significant differences from day to day, therefore, IOP measurements with and without latanoprost eye drops were performed on seven consecutive days (Table 4). Upon the application of commercial eye drops containing 50 μg/ml latanoprost the intraocular pressure decreased by 3.24 mm Hg from an average baseline value of 27.62 mm Hg to an average value of 24.38 mm Hg, whereas the prototype latanoprost test sample PLTS-N containing only 19 μg/ml resulted in an IOP decrease of 5.87 mm Hg from an average baseline value of 27.30 mm Hg to an average value of 21.43 mm Hg.
Table 4: IOP values before (week 1) and during the application of commercial 50 μg/ml latanoprost eye drops (week 2), and IOP values before (week 4) and during the application of PLTS-N 19 μg/ml lathanoprost eye drops (week 5).
Discussion
Eye drops for the topical treatment of diseases are composed of an active pharmaceutical ingredient (API) with pharmacological, metabolic or immunological activity, dissolved or suspended in a vehicle. Potential functions of the vehicle include dissolving or suspending the API, stabilizing the solution during shelf-life of the eye drops and during patient use, prolonging the contact time between the API and the ocular surface, supporting the penetration of the API into the ocular surface, and enhancing the biocompatibility of the eye drops [7,8]. Most eye drop formulations are requiring additives, in particular surfactants to dissolve lipophilic APIs like latanoprost. Mucoadhesive polymers like HA are prolonging the contact time by increasing the viscosity, and can, moreover, adhere to the glycocalyx of the apical epithelial cells, thus promoting the contact between the API and the ocular surface. Penetration enhancers such as BAC or EDTA weaken the transcellular or paracellular epithelial barrier function, thus enhancing the diffusion of the API into the ocular surface.
For reasons of acute and long-term biocompatibility it would be desirable to use vehicles which exclusively contain substances naturally occurring in the eye which, moreover, support ocular surface homeostasis and minimize unintended side effects of the API. Previous clinical investigations have proven this potential for a preservative-free solution comprised of 0.15% v/w hylan A dissolved in isotonic phosphate buffered saline solution with pH 7.4 [34-36]. It could be shown that this solution used as a vehicle result in a stable solution of 20 μg/ml latanoprost. It is hypothesized that the extra amount of 8 μg/ml latanoprost dissolved in the vehicle in excess of the latanoprost solubility in water is attributable to the association of latanoprost with the polyanion hylan A. Surprisingly the novel vehicle resulted in one test person with ocular hypertension in a superior IOP lowering effect as compared to commercial latanoprost eye drops with significantly higher latanoprost concentration. This seems strong evidence in favor of the assumption that hylan A acts as an influx transporter for latanoprost into the eye. This observation will need to be verified in a larger number of test subjects.
Beyond that, it will be of interest to investigate, if hylan A can also be used as an influx transporter for other APIs in the treatment of ocular diseases, eventually including retinal diseases, and if it can eventually contribute to a lower API concentration in eye drops, thus ameliorating intrinsic unintended side effects of the API, and, moreover, lowering manufacturing costs. The mode of action of hylan A as a transporter is so far unclear. Beyond stabilizing the epithelial barrier function, acting as an anti-inflammatory agent, and promoting intimate prolonged contact of latanoprost to the ocular surface, it is hypothesized that hylan A as a transporter with latanoprost associated, may bind to mucin MUC16 in the glycocalyx of the apical epithelial cells; may bind to adhesion molecule CD44 and to the receptor for hyaluronic acid-mediated motility (RHAMM) on the apical surface of the corneal and conjunctival epithelium; increases local tissue hydration, enabling temporal cell detachment that may create passages allowing for cell migration and transport of bioactive agents along the paracellular pathway; and binds to the HA receptor for endocytosis (HARE) on the apical surface of the corneal and conjunctival epithelium, causing HAREmediated endocytosis of the bioactive agent into the cytoplasm of the epithelial cells. The latter would be a novel option to transport bioactive agents with hylan A molecules as a transporter through the cell membrane of epithelial cells without damaging the cell membrane. In combination with the ability of hylan A to ameliorate the negative effects of corneotoxic substances, and lower concentration of API in eye drops, it is anticipated that patients will benefit from this novel vehicle and transporter in ocular diseases requiring long-term treatment.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.