Restorative Bio-Compatible Prosthetic Epoxy-Zeolite Composites: Strength and Resistance
Introduction
The injection of inorganic binders into the epoxy resin is a
known method of obtaining coatings, adhesives, compounds and
repair compositions. Epoxy compositions with building binders are
used as the basis for the production of self-leveling floors, repair
compositions and industrial compounds [1-22]. Attempts to use
epoxy compounds for implantation and exo-prosthetics [7-9,23,24]
have become popular recently. Epoxy glass and carbon plastics, as
well as composites with biocompatible fillers, were able to compete
with traditional metal and other (ceramic, wood) materials. In
particular, due to porosity, nerves, oxones and body cells can grow
through the surface of epoxy materials (which is impossible for
tantalum and titanium implants) [23,24]. And in exo-prosthetics
(manufacturing of external prostheses), epoxy composites
have a number of advantages - in particular, the possibility of
manufacturing in the field, replaceability, ease [8,9,23,24]. An
interesting potential filler for biocompatible innovative epoxy
composites is zeolite and its varieties. There are researches on
the effect on epoxy polymers of montmorillonite [14,15,25], clays
and brick dust [3,16], aluminum oxide [10,20,21], pyrophyllite
[11], brick powders [3,16], natural zeolites [18], bentonite [22],
copper [26,27]. These issues are dealt with by teams led by
Danchenko & Barabash [19-21], Erdogan [18], Choi & Leе [15],
Burmistrov & Mostovoy [16], Starokadomsky [3,10], Borisov and
others [25], Kahramanov & Allahverdieva [26,27]. The aim of the
work was to establish the possibilities of zeolite as a potentially
enhancing eco- The Composites Department in Chuiko ISC has 30
years of experience in polymer research with alumina and silica,
as evidenced by our works [1-13,19]. Also, we have experience
in the creation of prosthetic [5,8,9] and biocompatible [7,11-13]
compounds and fiberglass compatible and cheaper epoxy filler. The
selected concentration of 50 wt% is practically convenient because it
forms a convenient viscous composition, and also allows you to
mix components without precision scales (which is important in the
field conditions). At the same time, epoxy-ceolite-copper composite
was investigated in order to further create electro- and thermally
conductive materials for electronics and medicine (prosthetics).
Figure 1 Pilot, Hero of the Soviet Union A. Maresyev (noncolored photos), in 1943-1946, on a military plane, flew 86 sorties with prostheses instead of amputated legs. Сolored photo - examples of modern prostheses (see [28,29] and Wikipedia.org). Prosthetics has a long history. Even medieval Caribbean pirates also often had wooden legs and iron hooks instead of hands and fingers, and there have been attempts at dental prosthetics since primitive times. However, real shifts in science and practice began in the last century. Already in the 2nd World War, there are vivid examples of the effectiveness of prosthetics for maintaining extreme loads. So, the Soviet pilot A. Maresyev (Figure 1), after being wounded and amputated of his legs, returned to the sky and flew with prostheses. In total, during the war, he made 86 sorties, shot down 10 enemy (german) aircraft: three before being wounded and seven after. A. Maresyev (1916-2001) lost his legs at the age of 37, but with prostheses he lived an active life for another 59 years, and died almost at 90 years old in the new XXI-th century. He became the prototype for the hero of the soviet bestseller “The Story of a Real Man” [28,29].
Figure 1: Pilot, Hero of the Soviet Union A. Maresyev (non-colored photos), in 1943-1946, on a military plane, flew 86 sorties with prostheses instead of amputated legs. Сolored photo - examples of modern prostheses (see [28-29] and Wikipedia.org).
Methods and Reagents
Required Reagents and Method of Obtaining Composites
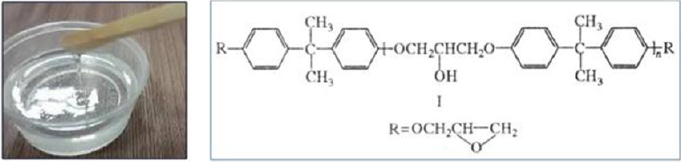
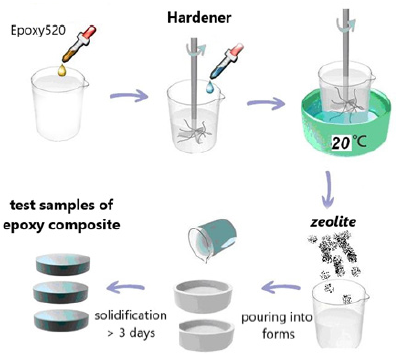
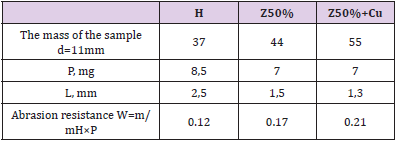
The Czech cold-hardening epoxy resin “Epoxy520” (Figure 2) was used for the works, which was cured with PEPA polyamine in a ratio of 5:1. The filler was added immediately after mixing these components, after which the composition was homogenized and immediately formed into samples (Figure 3, Table1). As a filler were used nano- and micro-sized zeolite particles (manufactured by PE “Eco Instinct”) for which sieving up to 100 μm was performed. Its supramolecular structure provides for the presence of micropores and internal nanopores (Figure 4). Visually, it is a gray powder converting the epoxy composition to a viscous ocher mass (Figure 4). Curing them gives wood-like biocompatible composites (Figure 5). Of the strength properties, we were interested in those that practically determine the applicability of these compositions. These are adhesion, strength (and modulus of elasticity) in compression, bending and abrasion, microhardness. Determination of strength and stability was performed according to methods corresponding to standards (GOST or ASTM). Shear adhesion tests (GOST 14760- 59) were performed on steel tear plates with a bonding area of 3cm2 and are presented in kgf. The corresponding value of adhesive strength can be obtained by dividing the obtained index by three. For compression tests (taking into account GOST 4651- 2014; ISO 604: 2002), cylindrical samples (diameter d= 6.5mm, height h=12 +-1mm) were taken and compressed on a Louis Shopper press machine until complete destruction. The machine provided compression of the sample with a given constant speed of movement of the active gripper, measurement of the load with an error of no more than ± 5% of the measured value. According to the test results, the strength was calculated: f = P / s (P - load in kgf, s - area equal to 0.332cm2) and module E: E = f / e (e - elongation equal to the length of the rectilinear section of the diagram in cm, divided by 10). Abrasion was performed by passing composite cylinders (diameter 6.5cm) on the surface of emery P60 at a distance of 10cm in both directions 40 times. Weight loss in mg and mm was determined. Abrasion resistance was calculated as the inverse of the abrasion mass of the sample. Abrasion resistance was determined by the empirical (derived from experiments) formula W = 1 × (m / mH) / P = m / mH × P, where mH / m characterizes the increase in mass (density) of the sample after filling.
Figure 4: View of zeolite powder (A), zeolite structure (B), epoxy-zeolite (C) & epoxy-zeolite-Cu and (D) compositions
Figure 5: View of polymerized composites - epoxy-zeolite & epoxy-zeolite-Cu with (A) and without zeolite (B)
Strength of Composite Materials
Adhesion Strength of the Epoxy-Composite to Steel
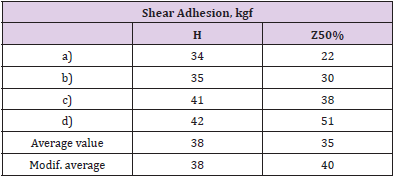
Shear adhesion to steel tends to be enhanced after the addition of 50% by weight of the zeolite. This can be seen from Table 2, from the results of which the most informative are the maximum obtained values (Table 2 d) and the modified average value (not taking into account the smallest indicators out of the series - in this case a) for C50%).
Mechanical Abrasion of the Epoxy Composites
As can see (Table 3), the injection of fillers leads to a marked increase in abrasion resistance. This is also noticeable while estimating abrasion in millimeters: the filled samples lose much less in size than the H-polymer sample. The increase in abrasion resistance (especially after the addition of copper, table 3) is quite natural, given the abrasive resistance of inorganic filler particles. Here need to notice also that the density of the sample naturally increases, especially after the addition of copper - even more (see the masses of the samples, Table 3).
Compressive Strength and Young Modulus of Composites
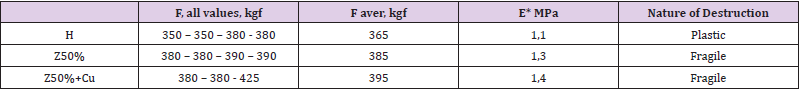
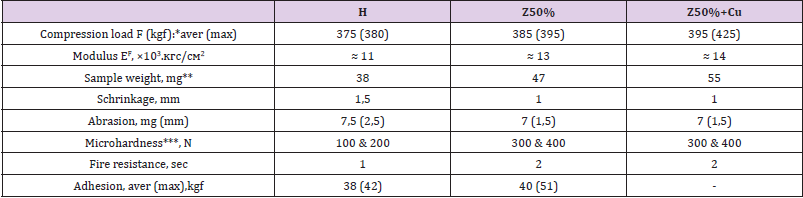
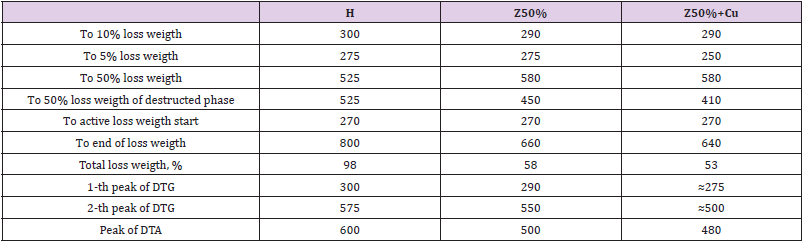
Table 4 demonstrate that 50 wt% zeolite makes it possible to increase the compressive strength and Young’s modulus. When copper powder is added to the composition, the effect is enhanced - in particular, the maximum value obtained is increased by 20% (425 instead of 380) compared to the H-polymer. The filling also changes the nature of the destruction from plastic (the sample crumples like plasticine) to brittle (cracks along the puncture lines). We can notice from Table 5, hat process of filling gives approximately double growth of microhardness. However, the filling increases the fragility of the samples - they crack already when hemispherepunch immersing to 20μm (Table 5) – while unfilled withstanding immersion up to 60μm. From the summary Table 6 you can see the overall effect of zeolite on the mecha parameters of polyepoxide. From Table 6 it is seen that the introduction of 50 wt% zeolite makes it possible to increase the strength and Young’s modulus under compression, microhardness, adhesion and fire resistance. This reduces shrinkage (an undesirable component of polymer synthesis) and abrasion wear. The addition of micro-nanoparticles of copper sometimes enhances the effect of increasing strength (for example, in compression).
Table 4: Values of load F in compression (cylindrical specimens with a diameter d=6.5cm, height 11-12cm), Young’s modulus E (*estimated) and the nature of the destruction of cracks when gripping.
Table 5: Microhardness (in H) of the surface of the composites, when the hemisphere is immersed by 10-30 microns. The # symbol indicates brittle cracking of the sample at the time of testing.
Table 6: All investigated parameters of strength of composites (* - aver (max) means Average and Maximal obtained value; ** - cylinder at h = 11mm, d = 6,5mm; *** - at 10 and 20μm immersion of steel hemisphere).
Resistance and Swelling of the Epoxy Composite in Aggressive Environments
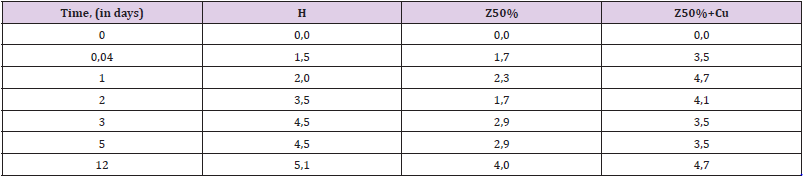
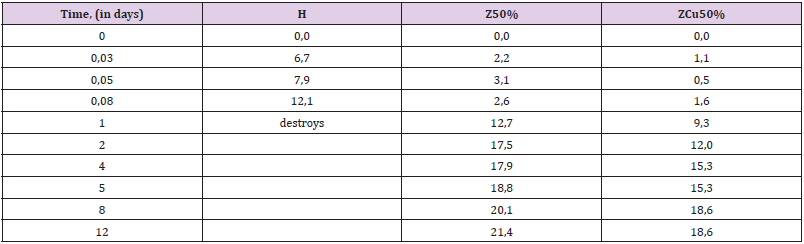
Endurance in 60% H2O2: Hydrogen peroxide, even at low concentrations (2-5%) is a substance quite aggressive against polymers (which is why it is used as a bleach in cosmetics). When the concentration of H2O2 reaches 30-40% and especially 50- 60%, its destructive effect is multiplied. At concentrations of 50- 60% peroxide easily corrodes organic tissues (in particular, forms severe burns on the skin). Polyepoxides are not significantly stable in 50-60% hydrogen peroxide. Therefore, the dynamics of their swelling and destruction in it can be tracked quite quickly (within 1-2 weeks) - in contrast to many other aggressive sopluk (acids, gasoline, alkalis). This makes the peroxide a convenient medium for evaluating the effect of the filling on the stability of the composite. From Table 7 it can be seen that the zeolite gives the composite greater resistance to peroxide. This can be seen from the decrease in the degree of swelling at all stages of exposure. The addition of copper powder in the composition somewhat weakens the positive effect of the filling, bringing the swelling index to that for the unfilled (H) sample (Table 7, Figure 6).
Resistance in a Mixture of Acetone: Ethylacetate (1 : 1): Acetone and acetone-contained solvents are very aggressive media for polyepoxides [3-9]. The unfilled polymer (especially freshly made) destructs in acetone solvents in a matter of days and sometimes hours. At the same time, the filling of epoxides can significantly increase their resistance to these mediums [3-11]. This can be seen from the results obtained. The unfilled sample is strongly swollen already in the first hours of endurance, and at the end of 1 day of endurance completely destroys (scatters in solvent). After the introduction of zeolite, the charge does not destroy, and swells much more slowly than the H-polymer. The addition of copper powder enhances this effect (Table 8).
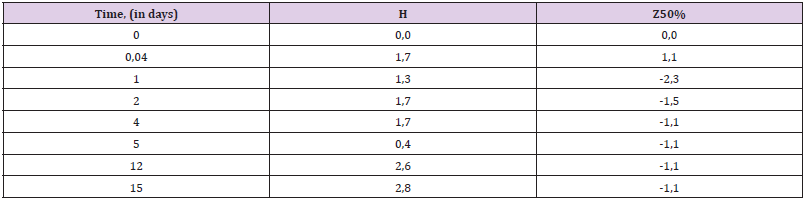
Water Absorption of Composites: Polyepoxides are generally quite stable in water. A water absorption rate of up to 1-2% during the month of exposure is considered normal. However, often even this figure needs to be improved. In addition. Epoxy products (coatings, parts, structures or ships) are often in the water for many years, and they need high water resistance. From our research we can conclude that the addition of zeolite makes the composite somewhat more resistant to water in the first half of the exposure (Table 9). Filling even causes the effect of slight weight loss, which can be caused by the phenomena of leaching of substances from the structure of the zeolite filler.
Thermo-Oxidative Destruction of Polymer Powders without and with Filler
A significant increase in fire resistance (Table 6) gives reason to expect certain changes in resistance to destructive thermal oxidation (DTO). A typical thermogram of unfilled polyepoxide is presented in Figure 9H. It shows that the maximum weight loss due to destructive thermal oxidation (DTO) occurs at 300 оС. The temperature of the 5% weight loss of the H-polymer, respectively, is 275 oC, and 10% 300 oC (Figure 9H. TG curve). After that, the DTO processes are significantly slowed down, forming a cycle of primary destruction processes. Starting from 450 оС, secondary processes (called burnout of coke residue) are activated. They also have their peak activity but occur with a more active and stable (without sharp differences) dynamics of heat release and weight loss (Figure 9). Half (50%) of weight loss occurs at 525 оС. Secondary DTO processes are completed only at the approach of 800 оС, when almost 100% of the sample burns out (Figure 9H). With the injection of 50 wt% zeolite, you can expect some changes in the thermal oxidation of the sample. Indeed, the primary DTO is less active. Thus, on the DTG curve at least at -0.1 (whereas for H-polymer at -0.2) and the DTA curve does not rise above 0.8. The maximum of the primary DTO is shifted toward 290 оС, ie at a lower temperature than the DTO of the H-polymer. Also, at lower temperatures (than for H-polymer) there is a secondary DTO, which according to the DTG curve is completed much faster - at 670 оС (H-polymer at 780 оС). At the same time, only up to 60% of the sample burns out, obviously it is 50% of the polymer and another 10% of the zeolite mass. When adding 10-13 wt.% Micro nano-particles of copper in the composite with zeolite, the nature of the thermal decomposition changes markedly. Now we actually have a single DTO process that is made up of sequential processes. All destructive processes are completed in record time - up to 630 оС. From Figure 9 and Table 10 it is seen that the filling generally impairs the resistance of polyepoxide to thermal oxidative destruction.
Conclusion
1) The injection of zeolite (in the amount of 50 wt%) is promising for the production of bio-eco-compatible composites for industrial and biomedical use, with enhanced strength and stability characteristics. The addition of micro-nanoparticles of copper in the composition in some cases can enhance the quality and strengthening effects of polymerized composites.
2) It is experimentally shown that the introduction of zeolite can dramatically increase the stability in an aggressive solvent (a mixture of acetone: ethyl acetate). Unlike unfilled (which completely destroys in 1 day of endurance), composites don`t destroy in it, and swell much more slowly. Also, the filling (after 2-3 days of exposure) increases the resistance to swelling in highly concentrated (60%) H2O2 peroxide: after 5 days of exposure, the unfilled swells by almost 5%, while the composite with zeolite - less than 3%.
3) It is established that the zeolite filling gives a double increase in microhardness and a slight increase in compressive strength and modulus. The addition of micro-nanoparticles of copper sometimes enhances the effect of increasing strength (for example, in compression). However, the filling increases the fragility of the samples.
4) It is shown that the filling doubles the fire resistance (2sec, whereas unfilled - 1sec). Thermogravimetry shows that the filling does not increase the resistance to primary thermooxidation of the epoxy polymer (passes at 290 oC instead of 300 oC in the unfilled) but makes it less active. Also, at lower temperatures are the processes of secondary thermal oxidation (at 670 oC instead of 780 oC in the H-polymer). The positive effect of the filler is reflected in an almost twofold reduction in the burn-up mass (up to 50-60% of the sample instead of 98% in the unfilled one).
For more Articles on : https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.