Costs Associated with A COVID-19 Screening Test, Country Comparison Editorial
Introduction
The COVID-19 pandemic has created an unprecedented need for diagnostic testing. In early 2020, diagnostic manufacturers were still struggling to raise the capacity of the new COVID-19 test to a reasonable level, but due to limited supply and high demand, prices became a challenge to low- and medium-income countries [1]. COVID-19 screening tests are essential in tracking where countries are in terms of COVID-19 and how far they need to act to combat /and manage it. There are two types of tests currently being conducted, namely the COVID-19 Polymerase Chain Reaction (PCR) and Rapid Antigen Tests. The PCR tests whether a person has the virus, whereas the Rapid Antigen tests whether a person has developed antibodies against the virus, assuming they have previously contracted the virus and whether their immune system produced antibodies in response to the infection. In South Africa, a COVID-19 test is accessible in a public sector setting; however, in a private setting, the RT-PCR tests cost R850 and results are available within 24 hours. Rapid Antigen Tests cost R400 providing results within 15-30 minutes [2]. The CMS alleged that laboratory prices for COVID-19-tests were exorbitant and unjustifiable at R850 per test. As of 12th December 2021, the cost of the RT-PCR test had been revised to not more than R500. The reduction of 41% decline followed a complaint by the Council for Medical Schemes (CMS) to the Competition Commissioner that private laboratories were [3].
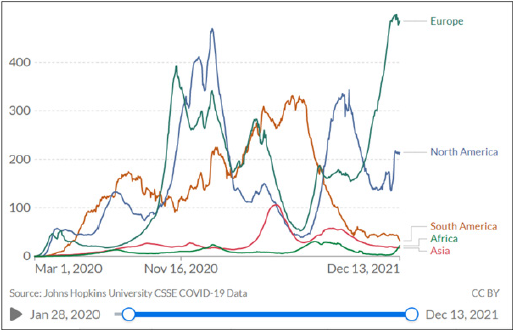
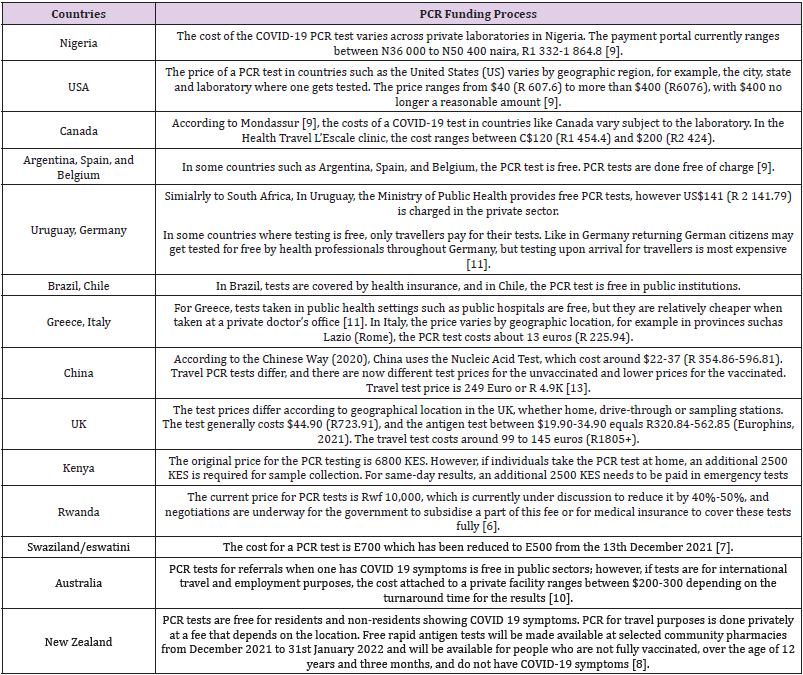
A COVID-19 test can be taken at a doctor’s room, in a laboratory with a medical prescription, a pharmacy, a screening centre, or even a hospital setting. The test is costly when it is carried out in private laboratories and the price varies per laboratory. The intervention by the CMS, which led to a price reduction, has been widely welcomed in the private sector. It has also been accepted as a victory for patients utilising private laboratories, especially those patients would require more frequent testing as the variants emerge ad mutate differently. Real-time- PCR is the most accurate diagnostic test for COVID-19, as it is more reliable than a rapid antigen test because of its high sensitivity and specificity to the virus. The PCR tests, which require a small saliva sample, have a sensitivity of 94% and specificity of 100%. In contrast, Antigen tests, which detect viral surface proteins, can provide a rapid and accurate indication of active infection, and provide a sensitivity of 97·1% and specificity of 98·5% [1,4]. Disparities in the price of a COVID-19 test vary by country, as shown in Table 1. COVID-19 as global pandemic has affected all countries across the globe, the recent new variant omicron has servery affected countries such as Europe a depicted in figure 1. Testing and tracing are one of the main strategies used to screen patients infected by the virus. A pandemic such as COVID-19 is financed mainly by governments through national budgets allocated to the ministries of health. Public sector testing is free of charge. However, should patients choose to test in a private setting or laboratory, there are costs associated. As of 11 December, the National Institute for Communicable Diseases (NICD) reported a total of 20 176 391 COVID-19 tests that have been conducted , with the private sector accounting for 54% of all tests conducted [5-14].
Conclusion
This editorial showed varying costs associated with COVID-19 tests depending on the setting, geographic region, and country. High extremal values of COVID-19 tests costs in other countries indicate an urgent need to regulate prices associated with a COVID-19 test. The case study of CMS in South Africa is essential key learning for other countries on how stakeholders and consumers can intervene of fair practice, competition, product, and services relating to the pandemic can be scrutinised for fairness. This editorial further calls for transparency in all input’s costs associated with COVID-19 tests. This is to ensure that the private sector does not unduly benefit or employ profit driven approaches or practices during a pandemic such as COVID-19. Lastly, the review of costs associated with COVID-19 tests should be a function of an ever-changing environment coupled with increased demand for the product or service and the emergence of new variants which may well require patients more frequents testing.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.