Derived Biobased Catalyst from the three Agro Wastes Peel Powders for the Synthesis of Biodiesel from Luffa Cylindrical, Datura Stramonium, and Lagenaria Siceraria Oil Blend: Process Parameter Optimization
Introduction
Over-dependence on petroleum reserves for the supply of energy, increasing demand for energy, price volatility of fossil fuel, the monopoly in the crude oil market, energy crisis associated with technological advancement are indicators that the current quantity will not meet the mandate with time [1,2]. Therefore, countries (EU, Spain, North America, South America, China, Brazil, India, Argentina, Australia, Canada, Cuba, Colombia, France, Ghana, Kenya, Sweden, Singapore, USA, UK, Zimbabwe, Peru, Pakistan, Italy, Japan, Malaysia, Mali, Mexico, Iran, Ireland, Norway, Germany, etc.) around the globe have shifted attention to biodiesel due to its excellent environmental attributes, sustainability attributes, biodegradability, non-toxic, readily available, and reduction or elimination of over-dependence on fossil fuel [3-6]. Meanwhile, biodiesel potential feedstocks come from first generation biodiesel feedstocks, which associated with the use of edible vegetable oil (beniseed oil, soyabean oil, corn oil, canola oil, palm oil, sunflower oil, coconut oil, olive oil, linseed oil, peanut oil, corn oil, papaya oil, etc.), the second generation biodiesel feedstocks, which make use of non-edible vegetable oil and animal fat (Jatropha curcus oil, pongamia pinnata oil, waste cooking oil, yellow oleander oil, cotton oil, grease, tallow, rapeseed, castor oil, karanje oil, neem oil, fish fat, pig fat, rubber seed oil, etc.), and the third generation biodiesel feedstocks, which involve the use of microalgae, algae, fungi, bacteria, latexes [5,7-15]. Nevertheless, exploiting firstgeneration biodiesel feedstock leads to a major problem especially in the present world of food shortage [16]. On the other hand, the use of third generation biodiesel feedstock requires a large amount of water for algae productivity, significant fertilizer for algae growth, high production cost using current technology, the long time needed for conversion to biofuel, contenders with regional suitability issues, lack of energy-efficient product, variations in the biofuel quality, and monoculture issue. Therefore, non-edible feedstocks in second-generation biodiesel feedstock are the only secure and viable future for all through biofuel production.
Meanwhile, it has been reported that the use of mix/blend oil tends to improve the yield and the quality of biodiesel; hence, researchers have reported the use of different blend ratios of oil for biodiesel synthesis. Khalil, et al. [17] reportedly the used oil blend ratio of 40:60 for rubber seed oil and palm oil, with NaOH as a base catalyst. The study reported by Qiu, et al. [18] adopted a ratio of 50:50 for the mixture of soybean and rapeseed oil with NaOH as a base catalyst. Milano, et al. [19], combined cooking with Calophyllum inophyllum oil in the ratio of 75:25, with KOH f as a base catalyst, while Hadiyanto, et al. [20] combined waste cooking with castor oil in the ration of 1:0, 1:2, and 2:1, respectively. Falowo, et al. [21], reported a blend of Neem and rubber oil in a 60:40 ratio with a base catalyst developed from elephant-ear tree pod husk. The work recently reported by Falowo, et al. [22], adopted the ternary blend ratio for Honne-Rubber-Neem oil, with mixed catalyst from three agro wastes. Observation from the reports showed that only Falowo, et al. [21,22] used heterogeneous catalysts for the synthesis of biodiesel production via oil blend. This was due to the heterogeneous catalytic nature such as reusability, recyclability, less water usage, non-toxic, low cost, eco-friendly, and high purity of by-product over the use of homogeneous catalysts (NaOH/KOH) [16,23,24]. To the author’s awareness so far, no single report on the use of the API gravity ratio has been reportedly used for oil blend for biodiesel synthesis. Also, no report has ever derived a based catalyst from the mixture of three green wastes of Cucurbita pepo, Musa acuminate, and Citrullus lanatus unripe peels for catalytic application. Literature survey showed that the unripe Cucurbita pepo peels contain 27.85% calcium [25] while unripe Musa acuminate peels contained 57% calcium mineral [26], the calcium content found in Citrullus lanatus unripe peels was reported to be 43% [27]. Proper processing of the peels through drying, sieving, and calcination at a higher temperature above 550oC has been established as a way of improving the content of the calcium in the peels [28-30].
Hence, this work focusses on the synthesis of a mesoporous based catalyst from the mixture of Cucurbita pepo, Musa acuminate and Citrullus lanatus peels, applied it to transesterification of Luffa cylindrical - Datura stramonium - Lagenaria siceraria oil blend to biodiesel. Detail characterization of the catalysts developed was carried out using Scanning Electron Microscopy (SEM), Fourier Transforms Infrared Spectroscopy (FTIR), X-ray Diffraction Analysis (XRD), X-ray Diffractometer (XRD), and Brunauer-Emmett- Teller (BET-adsorption) to determine its catalytic potential. Process optimization of transesterification of oil to biodiesel was carried out via response surface methodology (RSM). The quality of biodiesel was determined, and the results were compared with ASTM D6751 and EN 14214 biodiesel recommended standard.
Materials and Methods
Materials
Matured, Luffa cylindrical, Datura stramonium, Lagenaria siceraria seeds were collected from the nearby location around the rural house, Omu-Aran, Kwara State with proper permission obtained from the landowner in Nigeria. The seeds were separated from the husks by sundried for two weeks (14 days), and then oven-dried to a constant weight. The husk Datura stramonium has started splitting even before oven dried. The separated dried seeds were further obtained purely by winnowing and then milled into powders of 0.30 mm particle sizes, kept in separate cleaned containers for further processing (oil extraction). Cucurbita pepo, Musa acuminate, and Citrullus lanatus peels were obtained from the fruits. The peels were washed with distilled water twice, sundried for five days (5 days), and then oven-dried in a DHG-9101- 02 oven at 80oC for 2 h to achieved constant weight. The dried peels were milled into powders, separated into small particle sizes using a mesh strainer (mesh size: 125 mm-20 μm) to aid calcination. The fine sieved powders Cucurbita pepo peels powder (CPPP), Musa acuminate peels powder (MAPP) and Citrullus lanatus peels powder (CLPP) were kept in crucibles for further processing (Calcination/ thermal treatment). The seeds were identified by the Adepoju T. F., and the sample of the seeds have been deposited in Chemical and Petrochemical Laboratory 2, Akwa Ibom State University, Nigeria with a deposition number LC2019 for Luffa cylindrical, DS2019 for Datura stramonium and LS2019 for Lagenaria siceraria. All chemicals used were of analytical grades and need no further purification.
Methods
Oil Extraction: Oil extractions from the powders were carried out using solvent extraction in 1 L soxhlet extractor apparatus. Since the heating mantle was designed to handle three-Soxhlet extractors at once, mass extraction was carried out simultaneously. The oils were extracted from Luffa cylindrical, Datura stramonium, Lagenaria siceraria powders using n-hexane as the solvent. The procedures were as follows: the powders were put in a muslin bag and inserted in a Soxhlet extractor condenser with n-hexane at the round bottom flask placed on the heating mantle. The reaction temperature was monitored at 70oC for a complete extraction period (1 h). At the end of the reaction, the residual cakes were kept as a supplement for animal feeds while excess n-hexane in oils was recovered using the rotary evaporator. The clean oils Luffa cylindrical oil (LCO), Datura stramonium oil (DSO), and Lagenaria siceraria (LSO) were collected and kept in separate containers for further treatment. Oil qualities were ascertained by determining the physical, chemical, and other properties of the oils through AOAC (1997) standard test methods.
Oil Blend: The blend is the acts of mixing two or more substances, either miscible or non-miscible. For oils proper mix, it is worthwhile to know that the action of oil always increased by mixing several oils, nevertheless, the order in which the oil must be mixed must be factor properly. Lighter oil with smaller molecules will produce less viscous oils with high volatility, but heavier oil and larger molecules produce high viscous oils with low volatility. Hence, to obtain a low viscous, low density, and high volatile oil, there is a need for oil mix in an accurate blend ratio to increase the synergistic effect within the blended oil. One must know the nature (heavy or light) of the oil before mixing. The extracted oil is defined with API gravity, API gravity greater than 10 indicated lighter oil and the oil floats on water, the value of API gravity lesser than 10 indicated heavier oil, and the oil sinks on water. The API gravity of oil is calculated using Eq. (1) [31].
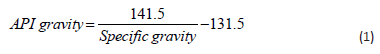
Oil Blend via API Gravity Estimate: The API gravity of the oils was estimated based on the specific gravity of the oil. The total API gravity of the oils was obtained from the API gravity of the oils obtained, the mix ratio of oil was computed using the mathematically derived Eq. (2), and the oil was properly mixed by heating at 50 oC on a magnetic shaker for 30 min.
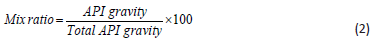
Catalyst’s Calcination and Characterization
The fine powders (CPPP, MAPP, CLPP), with mixed powder (30 g CPPP + 30 g MAPP + 30 g CLPP) (MP) were calcined at 650 oC for 3 h to obtain the powdered catalysts [6]. After cooling, the powdered were characterized using scanning electron microscopy (SEM), to examine the surface morphology of the catalysts, energy dispersive spectroscope (EDS) to determine the elemental analysis of the samples and the quantitative composition of the catalysts, X-ray diffractometer (XRD) equipped with Kά and Cu radiation source, accelerated at 20 mA and 30 kV, to establish the angular scanning electron performed in the range of 20o <2θ <80o at speed of 2oC min-1, Fourier transform infrared spectroscopy (FTIR), to check the presence of functional group and verify the presence of characteristic absorption bands of major elements present. The surface area and the basicity of the catalysts were examined using BET isothermal adsorption and the Hammett indicator method [32].
Synthesis of Biodiesel
The mixed oil (29:50:21) free fatty acid (FFA = 0.82<1.5) was within the moderate value for transesterification of oil to biodiesel [33]. Therefore, transesterification of mixed oil (MO) to biodiesel through methanolysis of the mixed powder (MP) was carried out using the procedure earlier reported by Adepoju, et al. [29] with few modifications. A three-necked-reactor was used to carry out biodiesel production, four factors with five-level were considered viz. reaction time, calcined mixed powder (CMP) amount, reaction temperature, and methanol/oil molar ratio (MeOH/OMR). The MO was preheated at 60oC for 30 min, a known CMP was added to 40 ml of methanol in a 250 ml flask, heated at 65oC for 20 min in a magnetic shaker, the insoluble methanolic-catalyst was transferred into the preheated oil in the reactor, and the reaction was monitored at a particular temperature until the reaction reach completion. At the end of the reaction, the solid phase catalyst was separated by decantation and the biodiesel phase was separated from the ethanol phase by separating funnel. The leached catalyst in the biodiesel was removed by washing with a solution (containing a mixture of 2.0 g of NaCO3 and 40 ml ethanol thermally heated for 2 h) under agitation. The mixture was filtered, washed with distilled water three times before the separation of biodiesel through gravity settling was carried out. The washed biodiesel was then dried over anhydrous Na2SO4 and then separated by filtration to obtain pure biodiesel. These processes were repeated based on experimental runs generated by response surface methodology experimental design.
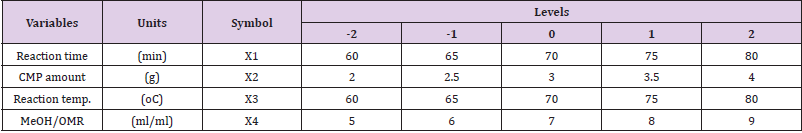
Experimental Design for Biodiesel Synthesis and Its Statistical Analysis: The four-level-five-factors used for biodiesel experimental designed with the respective units are presented in Table 1. A central composite design (CCD) was used which contains 24 non-center and 6 center points with high and low center factors, the design is a full type with 2 alpha and 1 block. A total of 30 experimental runs were obtained with central composite design replication occurred for every categorical combination. For analysis through the statistical approach, the model for response surface (biodiesel) and the interaction (variable factors) was evaluated by mean of fit summary. The model order, significant effects, and desired terms were evaluated by model effects. ANOVA was used to analyze the chosen model and view results, while diagnostic, evaluate the model fit, and also the transformation choice with the graph to interpret and evaluates the model. Moreover, process optimization was established by determining the probability value (p-value), the f-value (factor value), the degree of freedom (df), and the variance inflation factor (VIF), respectively. Linear regression parameters were obtained through evaluation of the coefficient of determination the predicted coefficient of determination, the adjusted coefficient of determination, and the adequate precision (Adeq. Prec.) to confirm the model suitability. Meanwhile, to show the relationship between two input variables and one output, three dimensional and contour plots prove to be the best representative of the correlation (Adepoju et al., 2016). The second-order polynomial model equation that further explains the relationship between biodiesel yield and the independent variables is mathematically expressed as Eq. (3).
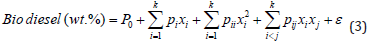
Where FAME is the response (biodiesel) in percentage, P_0 is the intercept, P_i is the linear coefficient, P_ii is the interaction coefficient, P_ij is the quadratic coefficient terms, X_i 〖 and X〗_j are the four factors and ϵ is the residual error.
Biodiesel Quality Characterization: The effectiveness and industrial application of biodiesel produce depend solely on its quality such as moisture content, density, viscosity, mean molecular mass, peroxide value, iodine value, saponification value, cetane number, higher heating value, API gravity, and diesel index. These properties were determined using AOAC, (1997) standard methods; the results were compared with biodiesel recommended standards [34,35]. All methods were performed in accordance with the relevant guidelines and regulations governing institutional, national, and international guidelines and legislation including the collection of plant material, the Experimental research and field studies on plants/seeds.
Results and Discussion
Extracted Oils Qualities and It Mixed Ratio
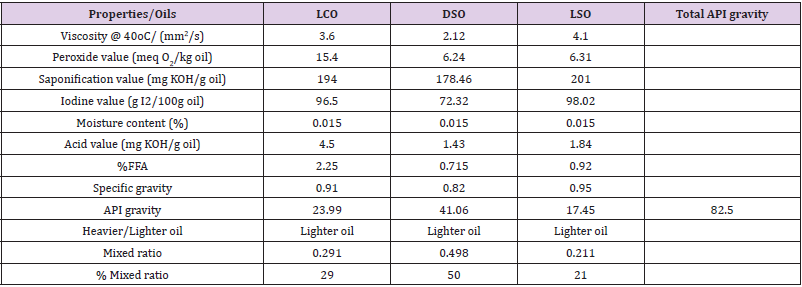
Table 2 showed the results of the qualities of the oils and the mixed oil obtained. From the table, it was observed that the specific gravity of the LSO (0.95) was highest, followed by the LCO (0.910), and then DSO (0.820). The higher the specific gravity, the lower the API gravity of the oil, and such oil tends to be less light in nature. The extracted oils were light oils with low free fatty acid value and low moisture content. The iodine value and the viscosity of the LSO (98.02 g I2/100g oil; 4. 10 mm2/s) appear higher than the value of LCO (96.50 g I2/100g oil; 4.10 mm2/s) and DSO (72.32 g I2/100g oil; 2.12 mm2/s). However, the acid value of the LCO (4.50 mg KOH/g oil) is higher than the acid value of LSO (1.84 mg KOH/g oil) and DSO (1.43 mg KOH/g oil), these showed that the LCO is non-edible oil. The API gravity of DSO (50) is greater than the values obtained for LCO (29) and LSO (21) which accounted for an oil mixed ratio of 29:50:21 for LCO: DSO: LSO. This mixed ratio produced oil with moderate FFA and of low density used for biodiesel production (transesterification).
Catalyst Characterization and Elemental Analysis
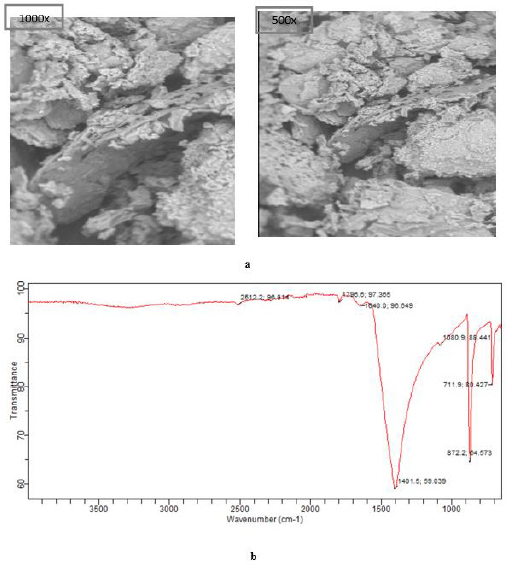
Scanning Electron Microscopy (SEM): Figure 1a showed the result of SEM images of morphological characteristics of the calcined mixed powder (CMP) at a different magnification of 1000x and 500x performed in the range of 20o<2θ < 80o at speed of 2oC/ min. The decomposed images after calcination at a temperature of 650oC for 3 h indicated non-uniform sizes with diverse shapes and slightly rough surface with cracks. This observation implied thermal treatment degrades organic substances in the mixed powder (MP) and also transforms the calcium carbonate (CaCO3) to the calcium oxide (CaO), owing to the gaseous form of carbon dioxide (CO2) which appears more porous, brittle and easy to ground.
Fourier-Transform Infrared Spectroscopy (FTIR): Figure 1b shows the FTIR spectra of CMP, distinct peaks were noticed at 711.9, 872.2, 1080.9, 1401.5, 1640.0, 1796.6, and 2512.2, respectively. The band at 1401.5 cm-1 represents bending vibration of the O-Ca-O group while the band at 771.9 – 1080.9 represents a stretch of CO32- molecules to higher energy value [3,36]. The spectrum stretches from 1640.0-2512.2 cm-1 indicated the presence of functional groups such as O-H, C-H for sp3 carbon, C=O for sp2 carbon, CHO, and N-H bond. Nevertheless, the undistorted assembly of CMP catalyst transformed into a spongy like structure signifies that calcination of the mixed powder at a temperature of 650oC was appropriate for a complete transformation of CaCO3 to CaO [16]. This observation could be attributed to the presence of functional groups present in the fruits during the growth period which occurred as a result of carbon growth inhibition.
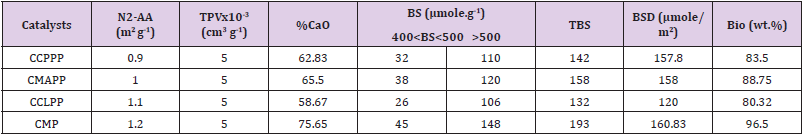
Brunauer-Emmett-Teller (BET): Table 3 represents the properties of the catalysts indicating the BET surface, total pore volume, basicity, and percentage of CaO converted through N2 adsorption-desorption isotherm Brunauer-Emmett-Teller (BET) analysis. Observation from the results shows the calcined mixed powder (CMP) has a high basic site than other calcined catalysts (CMAPP, CCLPP, and CCPPP) owing to the high CaO (75.65%) obtained during analysis in the calcined powder. The basic site density obtained for CMAPP (158. 00 μmole/m2) was higher than that of CCLPP (120.00 μmole/m2), but the value obtained for CCPPP (157.80 μ mole/m2) is approximately the same with CMAPP. However, the basic site density of the CMP (160.83 μ mole/m2) was the highest which produced the highest biodiesel yield during transesterification. The table also reflects the result of biodiesel yields, at the same process conditions, each catalyst was tested based on the CaO yield of the calcined powders (CCPPP: 62.83%; CMAPP: 65.50%; CCLPP: 58.67 and CMP: 75.65%), the yields of biodiesel based on the nature of catalyst showed the three calcined catalysts have a basic site for conversion of oil to biodiesel (83.50, 88.75, 80.32 (%wt.)), but the mixed calcined catalyst produced highest biodiesel yield (96.50% wt.), due to the percentage of CaO in the catalyst and high basic site. Hence, the catalysts could be an economic viable promising source for CaO catalyst production for industrial applications.
Optimization of Transesterification Mixed Oil to Biodiesel
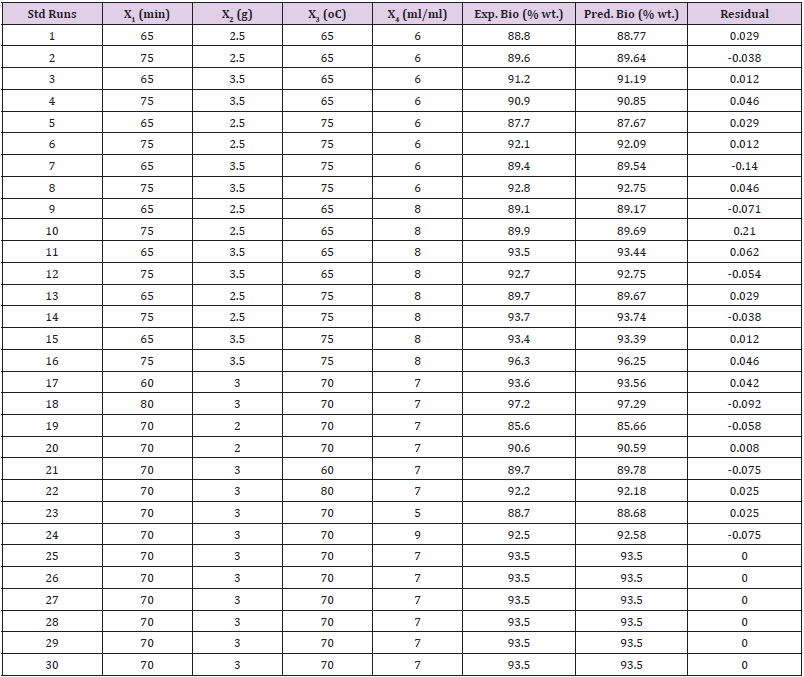
Experimental Results Analysis: Table 4 shows the coded experimental conditions, the experimental biodiesel yield, the predicted, and the residual values of the 30 standard runs generated by CCD. The table showed the maximum experimental yield of 97.20 (% wt.) at runs 18, while the minimum yield was obtained at runs 19 with a value of 85.66 (% wt.). Based on statistical analysis, the results were transformed into a fit summary, quadratic model, analysis of variance evaluation, diagnostics and graphs modeling, the predicted biodiesel yield of 96.63 (% wt.) was obtained at the following condition: reaction time of 80 min, CMP amount of 3.53 (g), reaction temperature of 90 oC, and CH3OH/OMR of 9:1 (ml/ml), at the desirability of 95.10%. This value was validated in triplicate, an average mean value of 96.50 (% wt.) was obtained which was close to the predicted value. The result proved that the methanolysis of CMP for the transesterification of mixed oil was successful.
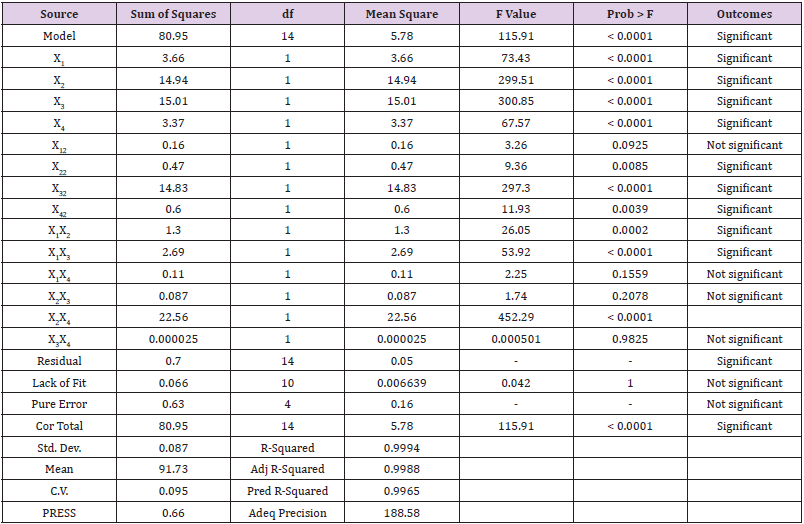
ANOVA and Fits Statistic: Table 5 shows the analysis of variance (ANOVA) for the response surface quadratic model and the Fit statistics. Observation from the table shows that the Model F-value of 1765.55 with a degree of freedom (df) of 14, implies the model is significant with prob value >0.0001. There is only a 0.01% chance that a “Model F-value” this large could occur due to noise. Meanwhile, values of “Prob > F” less than 0.05 show variable terms are significant. In this case, X1, X2, X3, X4, X12, X22, X32, X42, X1X2, X1X3, X1X4, X2X3, X2X4, and X3X4 were remarkable significant variable terms. The coefficient of determination is the correlation coefficient, also known as R-square,which allows it to display the degree of linear correlation between two variables. The value obtained in this study is high (99.94%), indicate a high degree of correlation between the interacting variables. The “Pred. R-Squared” of 99.65% is in reasonable agreement with the “Adj R-Squared” of 99.88%. The “Adeq Precision”, which measures the signal to noise ratio. Usually, a ratio greater than 4 is desirable, the ratio of 188.58 obtained in the study specifies an adequate signal.The polynomial model quadratic equation that shows the relationship between the biodiesel yield and the four-variable factors is presented in Eq. (4).
The final equation in term of coded
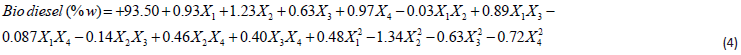
Meanwhile, the positive and the negative coefficients in the equation are the direct measure of the influence of variables on the response value. In this equation, the variable X2 with a coefficient of 1.23, f-value = 4796.50, with p-value<0.0001 is the most significant variable among the second-order polynomial equation in Eqn. (4).
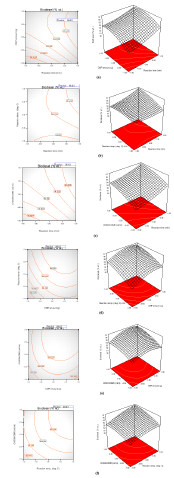
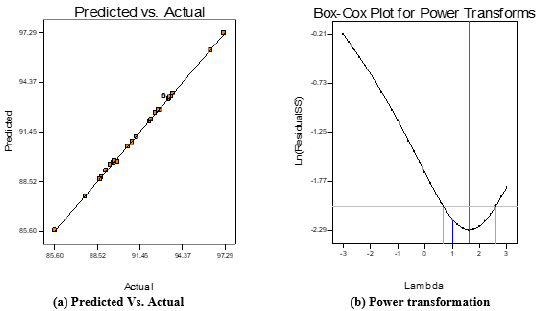
Graphical Plots: Furthermore, the relationship between the response variable (biodiesel) and the interactive variables (X1X2, X1X3, X1X4, X2X3, X2X4, and X3X4) can be represented in contour and the three-dimensional plots as displayed in Figures 2a-2f. The observation from the graph shows Figure 2e has the highest mutual interaction between CMP amount and MeOH/OMR on the response biodiesel yield. The mutual interactive effects between reaction time and MeOH/OMR (Figure 2f) is higher than that observed in the interactive effects noticed between reaction time and reaction temperature (Figure 2b) on the response, but the interactive effects in Figure2c (MeOH/OMR and reaction temperature) is lesser than the interactive effects showed by Figure 2b, but higher than the interaction between CMP amount and reaction temperature (Figure 2d). Meanwhile, the least interactive effects were noticed in Figure 2a, which was the interaction between CMP amount and reaction time on response biodiesel produced. In all, there exist perfect interaction among the variables, which confirmed the variable factors considered in this study play an important role in biodiesel yield. The relationship between the predicted biodiesel and the experimental biodiesel yields as well as power transformation boxcox were as illustrated in Figures 3a & 3b.
Catalyst Reusability Test
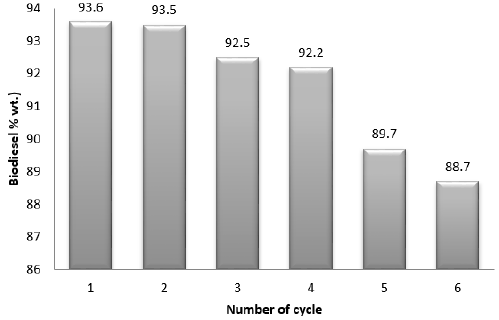
For catalyst purification and reusability analysis of the reaction mixture, a built-in heating system vacuum centrifuges operated at 3500 rpm was used, the recovered catalyst was washed with alcohol to remove the impurity at the surface of catalyst that occurred during the transesterification. The alcoholic washed catalyst was oven-dried at 100oC for 1 h and then cooled to room temperature before reused. Nevertheless, the reactor wall accumulates a 0.15 g catalyst during the reaction, which tends to reduce the surface area and lowered catalyst activity. Hence, the need for catalyst reusability tests on catalytic activities. Figure 4 shows that the catalytic activities-maintained stability from the 1st cycle to the 4th cycle with little decreased from 93.6 (% wt.) to 92.20 (% wt.). However, there was significant decrease observed in 5th and 6th cycles (89.7 and 88.7% wt.), this can be associated to the continuous intermediate products formed during the reaction, such as monoglyceride and diglyceride, which obstructed the catalyst holes as well as water to oxygen reaction that occurred at the catalyst surface, which reduce the catalyst sensitivity. Hence, catalyst reusability was stopped after the 4th cycle [37-39].
Properties of the Mixed Oil and Biodiesel
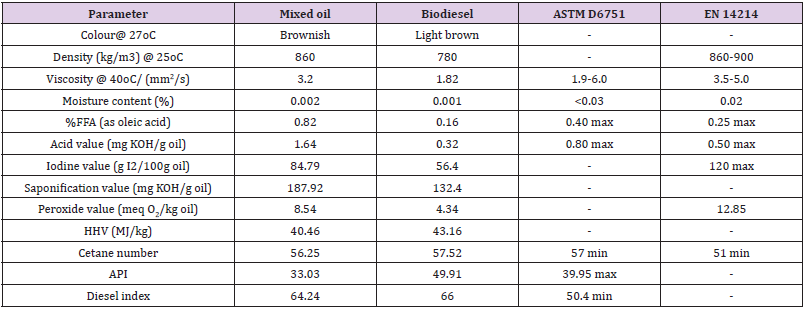
Method of AOAC were adopted to examine the properties of the blended oil and the product, the obtained results of the biodiesel were compared with the ASTM D6751 and EN 14214 biodiesel recommended standard (Table 6). It was observed that the decreased in the moisture content, density, viscosity, acid value, the iodine value, saponification, and the peroxide value of the blended oil to biodiesel was due to process transesterification. This confirmed that the synthesized product is consistent with biodiesel and the translation of blended oil complete transesterification reaction to biodiesel was achieved with insignificant resistance to flow and lessen internal drag in the engine. Further observation showed that the cetane number, the higher heating value (HHV), the API gravity, and the diesel index increased as blended oil was converted to biodiesel, this could be attributed to energy formation from viscous oil to low viscous oil. The high biodiesel yield obtained in this study could be attributed to due to decrease base consumption for neutralization. Based on cetane number, the higher the peroxide value, the better the cetane number and the decrease in ignition time [31,40]. The value of 4.34 meq O2/kg oil, can be attributed to the cetane number of 57.52 obtained. The higher heating value (HHV) of biodiesel is greater than that of blended oil which signify high heat of vaporization of water in the combustion of biodiesel. The American petroleum institute (API) gravity usually used to determine the weight of oil/petroleum in comparison with water, the value of 33.03 and 49.91 obtained for blended oil and biodiesel exhibited light oils. Diesel index which denotes the efficiency of the biodiesel as well as the ignition properties, the value obtained in this study were well within the required recommendation standard for biodiesel that can be used in I.C engine [41-44].
Conclusion
The study concluded that the ratio of blended oil produced low viscous oil, and the derived catalyst from the mixture of Cucurbita pepo, Musa acuminate and Citrullus lanatus peels powders, calcined at a temperature of 650oC for 3 h, produced high CaO- base (75.65%). Transesterification of blended oil to biodiesel was successfully carried out with maximum biodiesel yield of 97.20 (%wt.). A statistical software with second order model analysis predicted biodiesel yield of 96.63 (% wt.) at the following reaction time of 80 min, the CMP amount of 3.53 (g), the reaction temperature of 90 oC, and the CH3OH/OMR of 9:1 (ml/ ml), respectively. To validate this value, three experimental runs were carried out, and the average mean was determined as of 96.50 (% wt.) was obtained which was close to the predicted value. Analysis of variance test confirmed the significant of variables with p-value<0.0001. Catalyst reusability test was immobile at the 4th cycle due to loss of basicity that occurred due to leaching as a result of several recyclability during the reaction. Hence, the produced biodiesel conformed to biodiesel recommended standard, and the CaO catalyst could serve as promising economical feedstock for industrial application.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.