Endo-Parasitic Infestation in Captive and Free-Living Rhesus Macaques (Macaca mulatta) in Bangladesh
Introduction
Primates are noticed to be one of the severely threatened animals in the world, with approximately 50% of species currently at risk of extinction (IUCN [43]). More than 70% of primates are classified on the IUCN Red List as critically endangered, which in the near future they could disappear forever from Asia (IUNC [44]). Therefore, there is a growing recognition that it is very significant to better understand infectious disease dynamics in wild primate populations (Chapman, et al. [1,2]). Parasites play a significant role in the ecosystems, influencing the ecology and evolution of species interactions, host population growth and regulation (Hudson, et al. [3,4]) and even community biodiversity (Hudson [5]). Parasites and infectious diseases can trigger or accelerate population declines and have become a big alarm in conservation biology (Altizer, et al. [6]). Primates are susceptible to various protozoa and helminth parasites (Wanert, et al. [7]). Thus, parasites are an integral part of the natural history of mammals and are always of interest.
Natural infections of gastrointestinal protozoa and helminths have been exhibited in a variety of species of monkeys. Gastrointestinal parasitism in the colonies of non-human primates (NHPs) is often addressed as a research topic (Sano, et al. [8-10]). Strongyloides sp., Oesophagostomum sp., and Trichuris trichiura were previously considered among the most common pathogens causing poor development, anemia, and diarrhoea in macaques and other NHPs (Honjo, et al. [11-13]). Recently, captive NHPs were resulted pathogenic for their hosts and found that they can be frequently affected by several species of intestinal protozoans (Lee, et al. [14- 17]). Several studies have demonstrated that non-human primates may be carriers of human gastrointestinal parasites. Moreover, most parasites noted in NHPs represent a high zoonotic risk for researchers and caretakers in breeding centers (Loomis [18]).
Thus, people living in close proximity of such animals or individuals involved in game parks, animal orphanages or research stations may be at risk of acquiring pathogens from infected animals. Moreover, non-human primates are often valuable in studies of either human diseases processes or vaccine and drug development. It is therefore important that animals used in such studies should not harbour parasites since results obtained using parasitized animals may not reveal the true picture. Additionally, these animals play an important role in natural ecosystems; hence heavy parasite loads may have a negative impact on their population sizes. The rhesus macaque is well known to science due to its relatively easy upkeep in captivity, wide availability and closeness to humans anatomically and physiologically. It has been used extensively in medical and biological research on human and animal health-related topics. However, no previous works on endoparasitic infections of rhesus monkeys in Bangladesh were found in the literature. This article, therefore, highlights the exploratory and descriptive study on the different types of gastrointestinal parasites that affect populations of both wild and captive monkeys.
Materials and Methods
Sample Collection and Preparation
A total of 190 fecal samples were collected randomly from two study areas, the Shadhana Awshadhalaya factory area, Gandaria, Dhaka and the National Zoo of Bangladesh, Mirpur, Dhaka between March 2014 to February 2016. The monthly sampling covered the seasons of summer (March to June), fall (July to October) and winter (November to February). The samples of free-living monkeys were collected from Gandaria region and captive monkeys from Zoo with the permission of the authority. Only fresh, non-dry samples were collected and, whenever possible, when defecation was observed. As far as possible faecal samples were usually collected in the morning and then brought to the Parasitology laboratory of the Department of Zoology, University of Dhaka for microscopic examination.
Formalin-Ethyl Acetate Sedimentation Technique: Preserved faecal samples were processed using the formalin-ethyl acetate sedimentation technique (Cheesbrough [19]). Samples were collected into sealable, plastic bags immediately after defecation is observed. Within 8 h of collection, the collective sum of 2 g taken from each sample was transferred to 2-ml plastic tubes containing 0.75 ml 10% buffered formalin solution. Fixed faecal samples of Rhesus macaques were later transported to the Parasitological laboratory of the Department of Zoology, University of Dhaka, Bangladesh. All faecal samples were examined via a modified formalin–ether sedimentation protocol, substituting ethyl acetate for ether, as the former is less volatile (Hernandez, et al. [20]). Approximately 1 g of formalized faeces was diluted in 5 ml formalin, and centrifuged at 1,500 rpm for 3 min. The supernatant was then discarded, and the concentrated pellet was weighed (Muller-Graf, et al. [21]). To identify and quantify parasitic infection, an aliquot was removed from the homogenous suspension, placed in the chamber of a McMaster slide, and examined at 100x magnifications. It was counted all helminthes eggs and larvae were observed within the chamber’s 0.15-ml grid to calculate the number of eggs per gram of feces (EPG) from each sample.
Epidemiological Parameters: For each parasite, the prevalence and mean intensity were recorded. Prevalence was counted as the number of infected macaques per sampled subset of the population (Margolis, et al. [22-25]). Mean intensity was defined as the average number of infective stages (Table 1), among the infected members of each population.
Taxonomic Identification of the Parasites: Eggs of various gastrointestinal parasites were identified and the references and published articles such as Yamaguti [46-49], Cheng, et al. [26,27], other references, etc. were consulted. Representatives of each parasite were photographed by a digital camera.
Table 1: Average prevalence, intensity and EPG (egg/cyst/larva per gram) of parasites by location from March 2014 to February 2016.
Statistical Analysis: All statistical tests were carried out using SPSS (Version 22) and R software (Version 3.1.3). A nonparametric repeated-measures analysis of variance (ANOVA) was conducted. The variance ratio with the p-value was used to compare the dispersion of parasites between the Gandaria and Zoo samples. For each case, it was considered the parametric (Welch) and nonparametric (Wilcoxon) tests to study if the two population means for each of the parasites was the same for the two study areas. It was also found the 95% confidence interval of the differences between the two population means for each of the parasites.
Results
This paper aims to determine the parasite community of the host rhesus monkeys (Macaca mulatta), to estimate the prevalence and intensity of rhesus macaques in two study areas, the National Zoo (captive) and Shadhana Awshadhalaya factory area (freeranging) Dhaka, and to study the seasonal prevalence of helminth and protozoan parasitic infestation. Eggs, cysts and larva of parasites were identified based on their taxonomy, morphology and characteristics. Parasites belonging to 30 species were identified, 6 species of protozoa (Entamoeba coli, Eimeria sp., Isospora sp., Toxoplasma gondii, Chilomastix mesnili and Gregarina sp.), 5 species of cestodes (Taenia sp., Moniezia sp., Reillietina sp., Bertiella sp. and Amoebataenia sp.), 11 species of nematodes (Ascaris lumbricoides, Toxocara sp., Trichuris trichiura, Strongyloides sp., Ancylostoma sp., Ascarops sp., Gongylonema sp., Gnathostoma sp., Subulura sp., Enterobius sp. and Capillaria sp.), 7 species of trematodes (Neoglyphe sp., Watsonius watsoni, Schistosoma mansoni, Paragonimus sp., Clonorchis sinensis, Brachylaemus sp. and Gastrothylax sp.). The pentastomida (Linguatula sp.), was the first record in Bangladesh on the species of gastrointestinal parasites affecting monkeys in both captivity and the wild. This article compares the prevalence and intensity of parasites from two study areas which covered two study periods- the first study period (2014-2015) and the last study period (2015-2016).
Overall Estimate of Parasites Prevalence, Intensity and EPG (Egg/Cyst/Larva Per Gram): In the present study, from March 2014 to February 2016, the average prevalence and EPG of parasites were higher in Zoo than that in Gandaria. The average prevalence and EPG of parasites in Zoo were 46.42% and 358.89 respectively. The overall estimates of EPG prevalence and intensity of parasites for free-ranging and captive areas were averaged over the two study periods in both cases. It was observed that on average the number of eggs per gram of Gandaria samples was 306.67 and for that of Zoo, samples were 3888.89. While the average intensity of parasites was estimated to be around 35% in both the areas, the overall prevalence of parasites in Zoo (46.42%) was significantly higher (P-value <0.1) than Gandaria (39.76%) (Table 1).
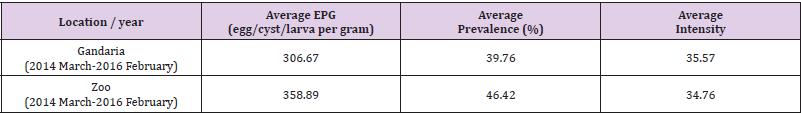
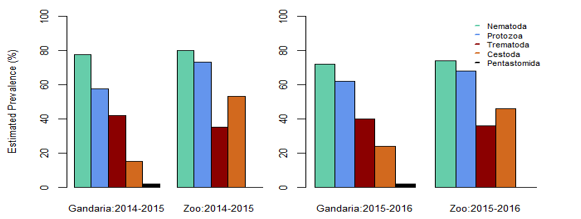
Estimated Prevalence, Mean Intensity and EPG (Egg/Cyst/ Larva Per Gram) of Parasite Groups by Location and Study Year: In the present study, it is evident that nematoda was the most dominant parasite. In both, the study areas, the prevalence of nematoda among others was the maximum during 2014-2015 and 2015-2016. In 2014-2015 samples showed the prevalence of nematoda was 77.78% in Gandaria and 80% in Zoo. In 2015-2016, the prevalence of nematoda in Gandaria and Zoo were 72% and 74% respectively. Unlike a very low prevalence (around 2%) of pentastomida was observed in Zoo (2014-2015). The study did not find any parasites of pentastomida in Gandaria. (Table 1, Figure 1). The study further observed the mean intensity of parasite groups among the two study periods and study areas. the estimated mean intensity of protozoa was maximum (76.06) in Zoo (2015-16). On the other hand, the mean intensity of pentastomida was absent in Zoo for both study years.
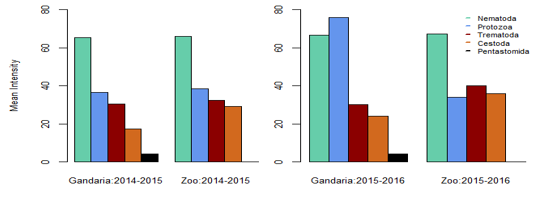
In Gandaria, the mean intensity of nematoda was 65.41 in 2014-2015 and that was 66.17 in Zoo at that time. In 2015-2016, the intensity of nematoda in both Gandaria and Zoo were 66.76 and 67.51 respectively. However, the mean intensity of trematoda and cestode (35.85) was found in Gandaria, Dhaka (2015-16) samples. (Table 1, Figure 2). The estimated EPG was the highest for nematoda in both study areas and both study periods. In 2014-2015, the EPG of Gandaria and Zoo were 879.17 and 791.67 respectively. Moreover, in 2015-2016 those were 970.83 and 937.50 accordingly. On the contrary, in 2014-15, the estimated EPG of trematoda was higher in Gandaria than that of Zoo and the opposite situation was seen in 2015-16. In the first study year, the EPG of cestoda in Gandaria was 50 and that was 138.89 in Zoo. On the other hand, in 2015-2016 those were 95.83 and 250 in Gandaria and Zoo respectively (Figure 3).
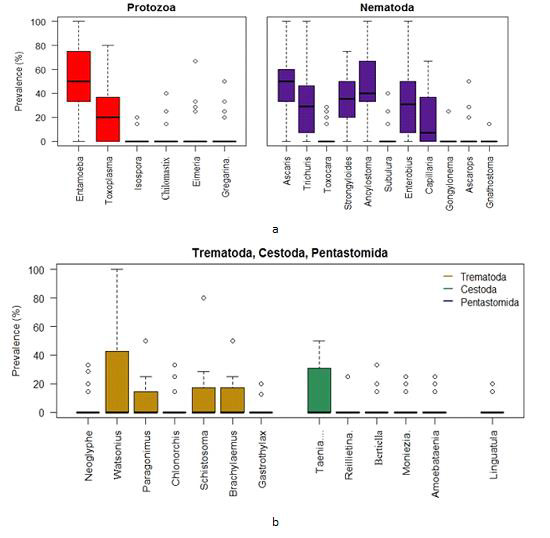
Box plots for the prevalence of parasites in the Gandaria samples (2 years data).
Using the combined data of Gandaria the Box plot for each of the parasites demonstrate that the prevalence of Entamoeba sp. was centered higher than all other parasites in the protozoa group. Both the Entamoeba sp. and Toxoplasma sp. showed higher spread and positive infections.The prevalence of Ascaris sp. was centered higher than other nematode parasites. Most of the parasites in this group were highly spread out. Four parasites in protozoa and five in nematoda group had very negligible prevalence (Figure 4a). The prevalence of most of the parasites of trematoda, cestoda and pentastomida were centered near zero. All most all the parasites occurred with less than 50% of prevalence in Gandaria. Only the Taenia sp. among the cestoda group had few non zero observations (Figure 4b).
Figure 4:
a. Box plots for the prevalence of parasites (protozoa & nematoda) in the Ganderia samples (2 years data).
b. Box plots for the prevalence of parasites (trematoda, cestoda & pentastomida) in the Gandaria samples (2 years data).
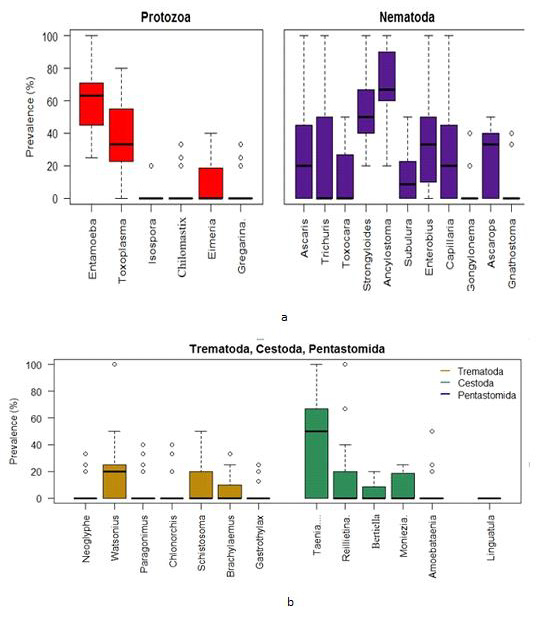
Using the combined Zoo data, the Box plots indicate that the prevalence of Entamoeba sp. was centered the highest followed by Toxoplasma sp. among the protozoa group. The Entamoeba sp. prevalence was negatively skewed while that of Toxoplasma sp. was positively skewed. The spread of Toxoplasma sp. prevalence was higher than other protozoan parasites. On the other hand, the prevalence of Ancylostoma sp. was centered the highest among the nematoda parasites. Most of the parasites in this group were highly spread out in terms of prevalence (Figure 5a).
Figure 5:
a. Box plots for the prevalence of parasites (protozoa & nematoda) in the Zoo samples (2 years data).
b. Box plots for the prevalence of parasites (trematoda, cestoda & pentastomida) in the Zoo samples (2 years data).
Discussion
The present analyses reveal significant richness and diversity of gastrointestinal parasites in the rhesus monkeys in Gandaria and Zoo. Parasites belonging to 30 species were identified, 6 species of protozoa (Entamoiba coli, Eimeria sp., Isospora sp., Toxoplasma gondii, Chilomastix mesnili and Gregarina sp.), 5 species of cestodes (Taenia sp., Moniezia sp., Reillietina sp., Bertiella sp. and Amoebataenia sp.), 11 species of nematodes (Ascaris lumbricoides, Toxocara sp., Trichuris trichiura, Strongyloides sp., Ancylostoma sp., Ascarops sp., Gongylonema sp., Gnathostoma sp., Subulura sp., Enterobius sp. and Capillaria sp.), 7 species of trematodes (Neoglyphe sp., Watsonius watsoni, Schistosoma mansoni, Paragonimus sp., Clonorchis sinensis, Brachylaemus sp. and Gastrothylax sp.) and 1 species of pentastomida (Linguatula sp.). This was the first record in Bangladesh on the species of gastrointestinal parasites affecting monkeys in both captivity and wild; and also their prevalence.
By comparison, 21 gastrointestinal parasites were identified in Kenya’s Tana River mangabey (Mbora, et al. [28]) and 14 parasite species were identified in monkeys of Uganda’s Kibale Forest (Gillespie, et al. [29]). Thirteen parasite species were found in Mahale National Park of Tanzania (Kooriyama, et al. [30]). Total 14 species were found in Rubondo Island National Park of Tanzania (Petrzelkova, et al. [45]) and a total of 23 gastrointestinal parasites were recorded in the Taï monkeys, African nonhuman primates (Kouassi, et al. [32]); recorded 23 gastrointestinal parasites with relatively high prevalence. However, no cestode species were recovered. According to the present work a total of 30 parasites with 5 species of cestodes were recorded. Jha, et al. [33] a prevalence survey of gastrointestinal parasites of Rhesus Monkeys was conducted in three temples Pashupatinath, Swyombhunath and Tripureshwor of Kathmandu. Total 121 fresh faecal samples, were collected randomly from these areas, and three species of protozoa and ten species of helminths were detected by microscopical examination of faecal samples.
A total of 30 gastrointestinal parasites were recorded in the present observation which represents the greatest parasite richness documented to date for Rhesus monkeys. Therefore, the diversity indices demonstrate considerable species diversity and equitable distribution of gastrointestinal parasites in monkeys. Faecal samples of monkeys (captive and free-living) were collected from the Shadhana Awshadhalaya factory area, Gandaria and the National Zoo of Bangladesh, Mirpur. Primates living in complex habitats are infected by a greater intensity and diversity of parasites (Poulin, et al. [34]; Nunn,et al. [35]; Nunn and Altizer [36]). Poor quality habitat and disturbed habitats are much more likely to harbor a greater intensity and diversity of parasites relative to host populations in optimum habitats (Poulin, et al. [34]; Nunn, et al. [35]; Nunn and Altizer [36]). In parasites with multi-stage life cycles, or with infective stages transmitted through the environment, the habitat of the host-parasite interaction plays a more direct role in the transmission of that parasite (Altizer [36]; Combes, et al. [38] and Grutter [39]).
Thus, the environmental conditions and management practices in the study areas (captive and free-living), such as food, cleanliness, overcrowding and deworming among others may influence the prevalence of parasitic infections and are responsible for the parasite richness in rhesus monkeys. In the present study, nematoda was the most dominant parasite in both study areas (captive and free-ranging). In contrast, the majority of the enteric parasites of captive non-human primates are protozoans, (Lane, et al. [40]; Ye,et al. [41]; da Silva Barbosa, et al. [10]). In this study, the average prevalence and EPG of parasites were higher in Zoo (captive) monkeys than that in Gandaria (free-living) monkeys. According to Kouassi, et al. [32], captive non-human primates are frequently infected with parasites having a direct life cycle and show a lower number of parasitic species in comparison with wild NHPs. Therefore, the environmental conditions of zoo and the life cycle pattern of parasites might be the reason for the higher prevalence of parasitic infections in Zoo than in Ganderia. According to Altizer, et al. [42], seasonality affects free-living stages of parasites through changes in temperature, humidity and rainfall, and host susceptibility and exposure through variations in physiology and behavior. Thus, environmental factors such as temperature, humidity and rainfall may influence the seasonal variation of parasites in Macaca mulatta.
Conclusion
This is the first report on endo-parasitic infection of the rhesus monkey in captive and free-ranging populations. The prevalence, EPG and intensity of helminth and protozoan parasites of the gastrointestinal tract in populations of free-ranging and captive monkeys were noted which provides baseline data on the gastrointestinal parasites in both study areas. The average prevalence of parasites was higher in captive monkeys than that in free-ranging monkeys. Seasonal fluctuation of parasitic infection was found from the captive and free-ranging monkeys which suggests environmental factors may affect the seasonal variation of parasites in monkeys. Rhesus macaques (Macaca mulatta) constitute an integral part of biodiversity and a cognizable link between humans and nature. Therefore, the findings of this study will serve as a guide to establish the necessary preventive measures to favor the conservation of this species.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.