Inhibitory Effects of Ninjin’yoeito Ingestion on a Reduction in Chewing-Like Movement and Cognitive Hypofunction in Powder Diet-Fed Mice
Introduction
“Mastication” refers to a series of processes from food ingestion until food mass formation through mixing with saliva. During mastication, the mandible rhythmically moves through cooperation among the teeth, jaw, and masticatory muscle. These movements are complexly and exquisitely regulated by the central nervous system based on various pieces of oral sensory information [1]. A recent study showed that mastication promoted the process of food digestion/absorption, exhibiting various actions: the activation of the brain, inhibition of brain aging, maintenance/improvement of the motor function, and regulation of blood circulation [2]. Thus, mastication is associated with various functions, playing an important role. However, recently, the influence of soft-diet-related masticatory hypofunction on biological bodies has been indicated [3]. Furthermore, a decline in the masticatory function (oral frailty) associated with aging-related tooth loss or oral hypofunction is considered to be an early, important sign of aging.
It increases the risk of dementia and promotes the risk of drymouth- or dysphagia-related aspiration pneumonia or a decline in the physical function (frailty) related to a reduction in energy intake. Many elderly persons have multiple diseases, and multi-component-system Kampo medicine formulations are usually used from the viewpoint of polypharmacy with high risk of adverse reactions [4]. Among these formulations, Ninjin’yoeito, which has been used in clinical practice, is effective for symptoms, such as a decline in physical strength after illness, fatigue/malaise, anorexia, night sweats, coldness of the hands and feet, and anemia. Recently, this formulation has been particularly prescribed for frail patients [5,6]. In this study, we examined the effects of Ninjin’yoeito on reduction in chewing-like movement and cognitive hypofunction using powder-fed mice.
Materials and Methods
Experiment Materials
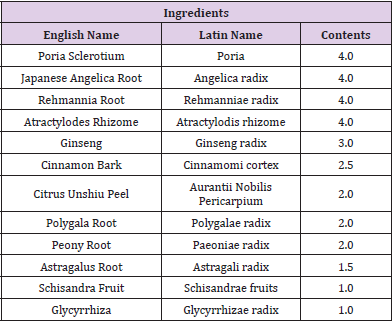
Laboratory pellet chow (solid diet) and powder chow (powder diet) were purchased from CLEA Japan (Osaka, japan). The dried extract powder of Ninjin’yoeito (NYT, Lot No. 1712111AO) was used in the present study and was manufactured by the GMP Pharmaceutical Factory of Kracie Pharma, Ltd. (Qingdao, China). NYT consists of 12 crude drugs: Rehmannia Root, Japanese Angelica Root, Atractylodes Rhizome, Poria Sclerotium, Ginseng, Cinnamon Bark, Polygala Root, Peony Root, Citrus Unshiu Peel, Astragalus Root, Glycyrrhiza, and Schisandra Fruit. The details are presented in Table 1.
Animals
Three-week-old male C57BL/6J mice were purchased from Japan SLC Inc. (Shizuoka, Japan), and maintained in a 12 h/12 h light/dark cycle in a temperature- and humidity-controlled room (room temperature, 23±2℃; humidity, 55±10%). The animals were divided into 4 groups. The animals were divided into 4 groups: solid diet group fed laboratory pellet chow (solid diet), powder diet group fed the powder chow (powder diet), and the two experimental groups which fed powder diet plus 1% NYT or powder diet plus 3% NYT. Animals had free access to food and water for 5 months. The body weight of each mouse was measured once a week and the total amount food and water consumed recorded 5 times per week. After the mice had been fed these diets for 5 months (at 25 weeks of age), blood was taken from each mouse by venous puncture using a heparin-containing syringe under anesthesia with isoflurane. Subsequently, the masseter muscle, temporal muscle, large salivary glands (parotid gland, submandibular gland, sublingual gland), gastrocnemius muscle, soleus muscle, and epididymal adipose tissue were extirpated, and the respective wet weights were quickly removed and weighted. The plasma was prepared and frozen at -40°C until analysis. This experiment was performed after approval by the Animal experiment committee of Kracie Pharmaceutical, Ltd. Kampo Research Laboratories following regulations on animal experimentation specified by the Research Laboratories and ‘Basic guidelines for the conduct of animal experiments in implementing agencies under the jurisdiction the Ministry of Health, Labour and Welfare’.
Measurement of Chewing-Like Movement
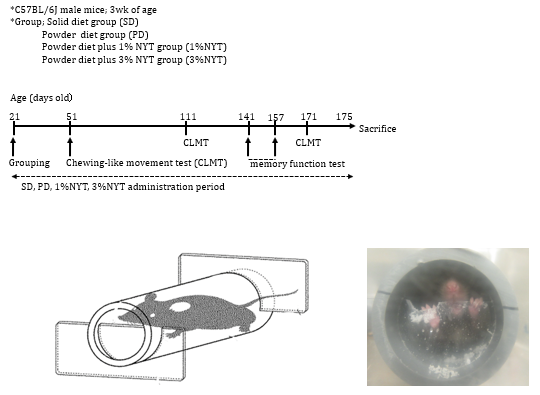
A device to measure chewing-like movement in mice was prepared, as described by Ayada, et al. [7], and the amount of chewing-like movement was measured, utilizing a mouse escaping habit by tail fixation (Figure 1); each mouse was placed in a thin cylinder, and plastic plates were set at the anterior and posterior notches of the cylinder. The murine tail was fixed with tape for a specific time, and the amount of change in the plastic plates was calculated. In this study, the measurement time was established as 1 hour, and the amount of change in the plastic plates was regarded as the amount of chewing-like movement in mice. The amount of chewing-like movement was measured 30, 90, and 150 days after the start of powder feeding (51, 111, and 171 days of age).
Figure 1: Experimental schedule for chewing-like movement and memory function of mice impaired by soft diet feeding.
Passive Avoidance Learning Test
The passive avoidance learning test was conducted from 120 days after the start of powder feeding (141 days of age). A grid was installed on the floor of an experimental device, and the space consisted of a white-light-illuminated bright room (9x25x32 cm) and dark room (30x29x32 cm). A plastic door was present between the two rooms. This test consisted of 3 parts: pre-training, training, and retaining trials. Pre-training trial (Day 120): Each mouse was placed in a bright room with the door closed and acclimated for 30 seconds. Subsequently, the door was opened. After the posterior limbs of the mice entered a dark room, the door was closed. The mice was acclimated in the dark room for 30 seconds, and collected from the device. Training trial (Day 121): Each mouse was placed in a bright room with the door closed. After 30 seconds, the door was opened. After the posterior limbs of the mice entered a dark room, the door was closed. After 3 seconds, electric stimulation (0.25 mA, 2 seconds) loading was conducted. The mice was collected from the dark room 30 seconds after electric stimulation, and placed in a breeding cage. Retaining trial (Days 122, 125, and 136): Each mouse was placed in a bright room, with the door closed. After 30 seconds, the door was opened. The latency time (cut off 300 seconds) until the posterior limbs or half body of the mice entered a dark room was measured.
Measurement of the Plasma NO levels
Plasma was centrifuged at 4°C and 14,000 g for 15 minutes using Amicon Ultra-0.5-PLGC Ultracel-10 membrane (UFC501096, Merck KGaA) for deproteinization. For measurement of the plasma NO level after deproteinization, a Nitrate/Nitrite Colorimetric Assay Kit (Item No. 760871, Cayman Chemical Company) was used.
Measurement of the Plasma Albumin Levels
For measurement of the plasma albumin level, an LBIS Mouse Albumin ELISA Kit (AKRAL-121, FUJIFILM Wako Chemicals) was used.
Statistical Analysis
The results are expressed as the mean±standard error (means±S.E.M.). Significance tests were conducted using Dunnett’s method with Statcel [3] software. P value of less than 0.05 was considered to indicate statistical significance. For the significance tests with serial data, repeated measure-variance analysis (repeated measure ANOVA) was performed. P value of less than 0.05 was considered to indicate statistical significance.
Results
Measurement of Chewing-Like Movement
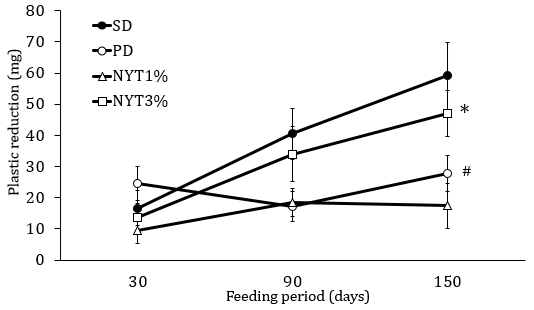
The amount of chewing-like movement was serially measured 30, 90, and 150 days after the start of powder feeding. As shown in Figure 2, in the powder diet group, the amount of change in the plastic plates was smaller than in the solid diet group, and there was a reduction in the amount of chewing-like movement. The powder diet plus 3% NYT group, significantly suppressed the reduction in the amount of chewing-like movement compared to the powder diet group, but the powder diet plus 1% NYT did not affect.
Figure 2: Effect of NYT on chewing-like movement.
Note: The amount of chewing-like movement was serially measured 30, 90, and 150 days after the start of powder feeding. The murine tail was fixed with tape for a specific time, and the amount of change in the plastic plates for 1 hour was calculated. Mice were divided into the following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT 3% group (NYT3%). Closed circle: SD; open circle: PD; open square: NYT 1%; open square: NYT 3%. Each value represents the mean ± S.E.M., n=12, #p<0.05 vs. SD and *p<0.05 vs. PD (repeated measure ANOVA).
Passive Avoidance Learning Test
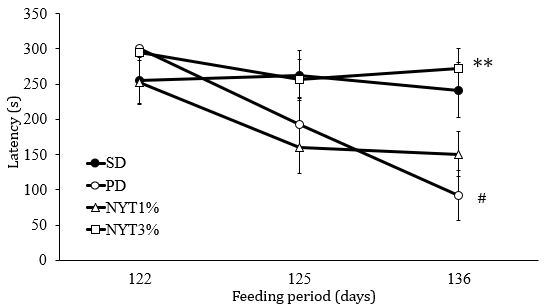
To measure mice memory function 120 days after the start of powder diet, the passive avoidance learning test was conducted. As shown in Figure 3, a retaining trial was performed 122, 125, and 136 days after the start of powder diet. In the solid diet group, memory was maintained throughout the test period, but in the solid diet group, the latency time until the mouse entered a dark room was shortened at Day 136, suggesting a reduction in memory. In the powder diet plus 3% NYT group, the latency time until entering a dark room was prolonged in comparison with powder diet fed mice; a powder-diet-related reduction in memory was inhibited.
Figure 3: Effect of NYT on memory function.
Note: Latency to enter the dark compartment before and after conditioning was measured in passive avoidance test. The cut off latency was set at 300s. Mice were divided into the following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT 3% group (NYT3%). Closed circle: SD; open circle: PD; open square: NYT 1%; open square: NYT 3%. Each value represents the mean ± S.E.M., n=12, #p<0.05 vs. SD and *p<0.05 vs. PD (repeated measure ANOVA).
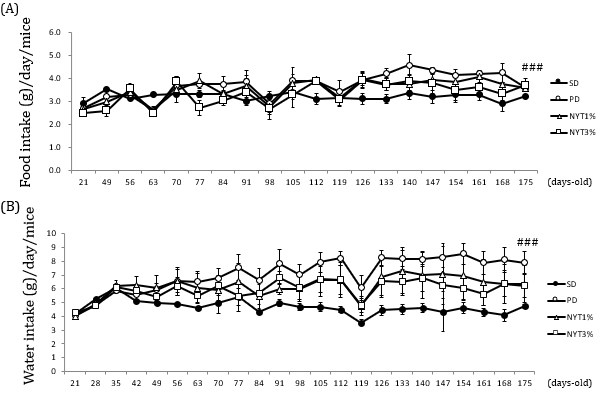
Body Weight/Food Intake/Water Intakes
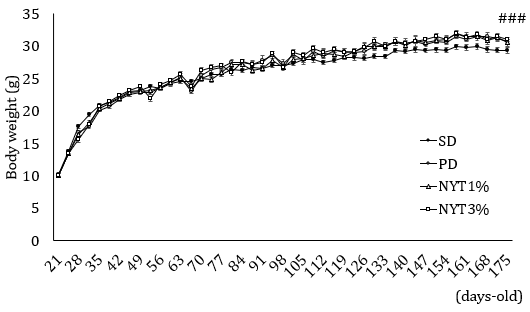
The body weight, food intake and water intake were serially measured. As shown in Figures 4 & 5, in the powder diet group, food/water intake increased in comparison with the solid diet group. In addition, weight gain was noted in the powder diet group but was not significantly different among the powder diet group, powder diet plus 1% and 3% NYT groups.
Figure 4: Effect of NYT on body weight.
Note: Whole-body weight was measured throughout the experimental period. Mice were divided into the following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT 3% group (NYT3%). Closed circle: SD; open circle: PD; open square: NYT 1%; open square: NYT 3%. Each value represents the mean ± S.E.M., n=11-12, ###p<0.001 vs. SD (repeated measure ANOVA).
Figure 5:
(A) Effect of NYT on food intake and
(B) Water intake.
Note: Whole food and water intake were measured throughout the experimental period. Mice were divided into the following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT 3% group (NYT3%). Closed circle: SD; open circle: PD; open square: NYT 1%; open square: NYT 3%. Each value represents the mean ± S.E.M., n=4, ###p<0.001 vs. SD (repeated measure ANOVA).
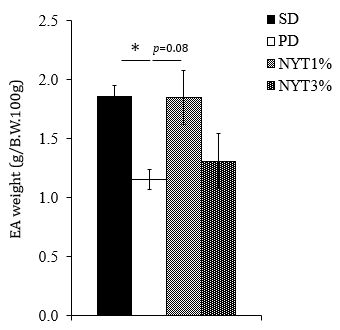
Wet Weights of Tissues
As shown in Figure 6, the epididymal adipose tissue weight significantly decreased in the powder diet group. Whereas, there was a slight increase in the powder diet plus 1% NYT group. However, there were no differences in the weights of masseter muscle, temporal muscle, gastrocnemius muscle, or soleus muscle among the groups (data not shown).
Figure 6: Effect of NYT on epididymal adipose tissue weight.
Note: Mice were divided into following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT 3% group (NYT3%). Each value represents the mean ± S.E.M., n=8-9, *p<0.05 vs. PD (Dunnett’s test).
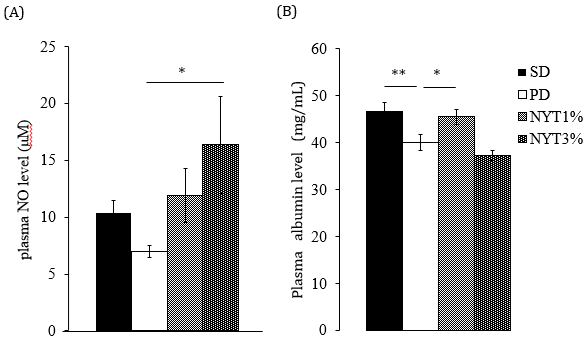
Plasma NO Levels
As shown in Figure 7A, the plasma NO level in the powder diet group was slightly lower than in the solid diet group. In the powder diet plus 3% NYT group, there was a significant increase compared to the powder diet group, and the plasma NO level was maintained at the same level as the solid diet group.
Plasma Albumin Levels
As shown in Figure 7B, the blood albumin level in the powder diet group was significantly lower than in the solid diet group. In the powder diet plus 1% NYT group, there was a significant increase.
Figure 7: (A) Effect of NYT on plasma NO level and
(B) Plasma albumin level.
Note: Mice were divided into the following 4 groups: solid diet group (SD), powder diet group (PD), powder diet plus NYT 1% group (NYT1%), powder diet plus NYT3% group (NYT 3%). Each value represents the mean ± S.E.M., n=8-9, *p<0.05 vs. PD (Dunnett’s test).
Discussion
Solid diet serially increased the amount of chewing-like movement in mice, whereas there was no serial increase during powder diet. 3% NYT-containing powder diet serially increased the amount of chewing-like movement, as demonstrated for solid feeding. However, there were no changes in the weights of masseter muscle, temporal muscle, or large salivary gland among the groups (data not shown). As the cause, it is possible that NYT administration contribute to the actions of other masticatory organs, such as the mouse jaw and mouth-opening muscle.
A reduction in masticatory movement reduces memory [8]. As nerves are extensively distributed in the brain area responsible for oral-tissue domination, so the mechanism of masticatory function and memory is complicated. Mastication-related mechanical stimuli from the oral area potentiate central nervous stimulation, leading to the activation of cholinergic neurons in the pedunculopontine tegmental nucleus, neurogenesis in the hippocampus, and increases in BDNF secretion and synapse transmission efficiency [9]. Furthermore, several studies indicated that masticatory movement increased intracerebral blood flow [10,11]. Recently, it was shown that an increase in cerebral blood flow was mediated by the nucleus basalis of Meynert [12]. This nucleus is known as a cholinergic nucleus, and NO released by the vascular endothelium may activate cholinergic nerves. In this study, powder feeding resulted in a reduction in memory on the passive avoidance test, and NYT administration improved it. Although cerebral blood flow was not measured in this study, NYT administration suppressed the decrease in blood NO level induced by powder feeding. Thus, it was suggested that NYT contribute to memory improvement by increasing cerebral blood flow through the enhancement of NO production. NYT consists of 12 crude drugs (Table 1).
Table 1: Composition (daily dosea) of Ninjin’yoeito Formulations.
Note: aApproximate 6700 mg of dried water extract of NYT was prepared in GMP-standardized factory of Kracie Pharma, Ltd. (Japan) on the basis of above described composition.
Ginsenoside Rb1, which is contained in ginseng, was reported to increase the NO level [13]. In addition, a study indicated that cinnamaldehyde contained in Cinnamon Twig and paeoniflorin contained in Peony Root exhibited NO- or inducible NO synthase (iNOS)-mediated anti-inflammatory or vasodilative actions [14]. Several studies reported the assessment of memory using the passive avoidance test in powder-fed mice [8,15]. Fukushima-Nakayama et al indicated that powder feeding reduced memory in mice and confirmed a reduction in the hippocampal BDNF level as a part of the action mechanism [15]. It was also reported that reductions in the hippocampal nerve activity, neuron count, acetylcholine level [16], and neuron density [17] involved in powder-feedinginduced memory impairment. Furthermore, in addition to the hippocampus, powder-feeding-related decreases in the cerebral cortex levels of BDNF [18], dopamine, and acetylcholine [19] were indicated. However, in this study, there were no significant changes in the mouse hippocampal BDNF level (data not shown). In the future, changes in the above neurotransmitters should be examined using the hippocampus and cerebral cortex to clarify the action mechanism of NYT.
Powder feeding increased food/water intake in comparison with solid feeding. With this, weight gain was noted. Several studies indicated that powder feeding slightly increased the body weight [20,21], and it suggests that the satiety center is not stimulated through a decrease in the opportunity of mastication, leading to weight gain in some cases. Mastication promotes saliva secretion, assisting digestion/absorption [2]. However, powder feeding decreased the fat volume, although there were no changes in the gastrocnemius or soleus muscle weights (data not shown), suggesting some change in nutrient absorption in mice. The blood albumin level, which reflects the nutritional status, significantly decreased through powder feeding in comparison with solid feeding, suggesting that intestinal nutrient absorption is reduced in the absence of mastication. Furthermore, the maintenance of plasma colloid osmotic pressure is known as the function of albumin, and when the plasma albumin level decreases, a specific blood osmotic pressure is maintained.
Therefore, clinical symptoms, such as edema and ascites, occur through extravascular water leakage [22]. From these results, powder feeding may have affected intestinal nutrient absorption, reducing the blood albumin level, influencing water metabolism, and resulting in a heavy body weight despite a small fat volume, that is, swelling or a flabby body. NYT administration increased the blood albumin level, with a slight improvement in the fat volume. A study indicated that NYT improved the small intestinal morphology and intestinal absorption function in old mice [23]. In this study, neither the small intestinal morphology nor intestinal absorption function of mice after NYT ingestion was measured. However, the albumin-level- and fat-volume-improving effects of NYT may be associated with improvements in the small intestinal morphology and intestinal absorption function of mice.
Conclusion
This study showed that NYT inhibited a reduction in chewinglike movement and cognitive hypofunction in powder-fed mice.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.