Healing and Prophylactic Efficacies of Topical Composite Herbal Medication on Overuse-induced Achilles Tendinosis
Introduction
Tendinopathy is a clinical condition characterized by pain and swelling in and around degenerative tendons [1]. Achilles tendon is vulnerable to developing tendinopathy, especially among athletes due to the continuous, prolonged and intense functional demands imposed on it during sports training and competitions [2]. Tendinopathy affects not only athletes but 25% to 30% of injured were non-athletes resulting in significant loss of working days [3]. Hence, this condition poses a heavy toll on the individual as well as the society by increasing workers’ compensation costs. Traditionally, Achilles tendinopathy was described as Achilles tendinitis which refers to an inflammatory process with the occurrence of pain and swelling at the tendon. With more scientific evidence gathered, there is better understanding to the condition that it is a degenerative process without histological or clinical signs of intratendinous inflammation [4,5], the condition is now described as Achilles tendinosis which is the outcome of imbalance between degeneration and synthesis of cell-matrix upon repetitive trauma [6]. However, the etiology behind Achilles tendinosis is still unclear but various factors have been reported to induce the condition which included overuse, decreased blood supply and muscle imbalance or weakness [7]. Essentially, Aström [8] reported a survey of surgical and histopathologic findings that 90% of biopsy specimens from symptomatic parts of tendons with tendinopathy exhibited symptoms of tendinosis including abnormal fiber structure, focal hypercellularity and vascular proliferation [8]. This suggested chronic degeneration of Achilles tendon would be a risk factor for rupture of the tendon. Hence, in addition to treatment upon occurrence of injuries, prophylaxis against development of tendinosis is important for reducing risk of tendon rupture as well.
Currently, majority of the studies on management of Achilles tendinosis focused on the therapeutic effects of the treatment protocols upon occurrence of the disorder. For instance, Furia [9] demonstrated that high-energy extracorporeal shock wave therapy (ESWT) was more effective in treating chronic insertional Achilles tendinopathy in terms of improved perceived condition of diseased Achilles tendon and pain score [9]. However, in a double-blinded randomized controlled trial by Costa et al, no significant treatment effect was found in 49 patients with Achilles tendinosis treated with low energy ESWT [7]. Nevertheless, a recent placebo-controlled randomized control trial which compared the application of pointfocused and line-focused applicator in the treatment of achilles tendinopathy, found significant improvement for all study groups (including placebo group) without a statistically significant benefit for ESWT groups during 24 weeks [10]. Besides, Stergioulas, et al. [11] reported that low-level laser therapy (LLLT) with eccentric exercise would decrease pain, morning stiffness, tenderness to palpation, and improve active dorsiflexion and crepitus with no side effects in patients with Achilles tendinosis as compared to those underwent eccentric exercise only [11]. In addition to physical treatments, healing effects of medications have also been studied. Paoloni, et al. [12] demonstrated that topical glyceryltrinitrate (GTN) significantly reduced pain, tenderness on palpation and improved ankle plantar flexor activities in subjects with non insertional Achilles tendinopathy [12].
Complementary to the western treatment approach, Traditional Chinese herbal medicine (TCM) has long been used for treating tendon and ligament injuries. Fu, et al. [13] demonstrated the ultimate strength of healing tendon was significantly promoted and collagen deposition was enhanced with better fiber alignment being observed in rat patella tendons after injection of total flavones of Hippophae rhamnoides, also known as Shaji [13]. Further, our previous findings suggested that topical application of Panax notoginseng extract coupled with therapeutic ultrasound would improve the strength of repairing rat ligament [14]. Although previous works had demonstrated the therapeutic potential of TCM on tendon repair, information on treatment effect as well as the prophylactic potential of TCM on overuse-induced tendinosis is scarce. Thus, the objective of the current study was to investigate the healing effects and prophylactic efficacy of a composite TCM extract on overuse-induced Achilles tendinosis.
Materials and Methods
Chinese Herbal Medication
The herbal application formula for this study comprised Dipsaci Radix (DR), Rhizoma Notoginseng (RN), Flos Carthami (FC), and Rhizoma Rhei (RR) in the ratio 1:1:1:1. These herbs were reported to control inflammation, stimulate circulation and promote fibroblastic activity for tissue repair [14-17]. After purchasing the raw herbs, their identities were authenticated using thin-layer chromatography with reference to the methods recommended by the Chinese Pharmacopoeia (2010). The herbs were crushed into small pieces and extracted with distilled water twice by heating under reflux followed by heating under reflux with 95% ethanol twice. The collected aqueous and ethanolic extracts were filtered and lyophilized to give the powder. The extraction yields of aqueous and ethanolic extracts were 40.0% and 5.0% respectively. The herbal paste was prepared by mixing 3.9g of aqueous extract and 0.5g of ethanolic extract in 3.4ml 50% ethanol-water. The herbal paste was applied topically at the location of Achilles tendon of both hind limbs and wrapped with Micropore surgical tape (3M, USA) (Figure 1). The herbal plasters were replaced on daily basis.
Animal Model
Forty 3-month-old female Sprague-Dawley (SD) rats were used in this study. The animals were randomly assigned into 4 groups, namely, Cage control (CC) which received no treatment, Exercise without herbal treatment (EX), Exercise with herbal treatment provided before forced running period (Pre-HB) and Exercise with herbal treatment provided after forced running period (Post-HB), with 10 rats in each group. Overuse-induced Achilles tendinosis was created in EX Pre-HB and Post-HB groups by an enforced running protocol [18]. Briefly, the rats were subject to run on a rat treadmill (Duen Production BCPT-98, Hang Zhou, Zhejiang, China) at 20m/ min with a 20-degree decline slope modified from the eccentric loading model of Soslowsky [19]. In order to increase loading on the rats’ hind limbs, the upper trunk of rats was suspended with a harness so that their forelimbs were lifted off and hence the rat ran in a bipedal pattern with the hind limbs. The rats were subject to the running exercise for 1 hour per day, 7 days per week for 8 weeks. During the 4 weeks before forced exercise period, Pre-HB rats were treated with herbal extract application to both Achilles tendons on daily basis whereas CC and EX rats were kept in cage without any treatment. Upon completion of the 8-week running program, the Post-HB rats were treated with herbal extract application to both Achilles tendons on daily basis for 6 weeks. Pre-HB, EX and CC animals were sacrificed by CO2 inhalation at the end of forced exercise period whereas Post-HB rats were sacrificed at the end of the 6-week herbal treatment period. Both Achilles tendons were harvested and kept frozen at -70°C until further testing. The protocol of the current study was approved by the Animal Ethics Review Committee of the administering institution.
Histological Analysis
The left Achilles tendons were used for histological analysis. The specimens were rehydrated in 0.01M phosphate-buffered saline (PBS) containing 20% sucrose for 30 minutes at room temperature. Then, the tendon samples were sectioned at a thickness of 7μm with a cryostat followed by hematoxylin-eosin (H&E) staining. The sections were examined using light microscope (Model: Eclipse 80i, Nikon, Japan) immediately after staining. Images of the sections were digitally captured with a camera (Model: SPOT Flex 15.2, 64Mp, SPOT Imaging Solutions, Sterling Heights, MI, USA) mounted on the microscope at 100x magnification. The captured images were further analyzed using ImageJ version 1.46r (NIH, USA). The level of cellularity was estimated based on the density of cell count in the area of tendon captured.
Biomechanical Testing
The left Achilles tendons were used for histological analysis. The specimens were rehydrated in 0.01M phosphate-buffered saline (PBS) containing 20% sucrose for 30 minutes at room temperature. Then, the tendon samples were sectioned at a thickness of 7μm with a cryostat followed by hematoxylin-eosin (H&E) staining. The sections were examined using light microscope (Model: Eclipse 80i, Nikon, Japan) immediately after staining. Images of the sections were digitally captured with a camera (Model: SPOT Flex 15.2, 64Mp, SPOT Imaging Solutions, Sterling Heights, MI, USA) mounted on the microscope at 100x magnification. The captured images were further analyzed using ImageJ version 1.46r (NIH, USA). The level of cellularity was estimated based on the density of cell count in the area of tendon captured.
Biomechanical Testing
The right Achilles tendons were tested for their biomechanical properties. Each specimen was dissected by leaving only the intramuscular tendinous fibers, Achilles tendon and calcaneus intact. The intramuscular tendinous fibers were then secured between 2 plastic strips with epoxy glue (Aron Alpha, Toagosei Co. Ltd, Columbus, OH) and mounted onto the cross-heads of a material testing machine (Model: Synergie 200, MTS System Corporation, Ivry sur Seine Cedex, France). An extensometer (Model: 634.12F- 24, MTS System Corporation, Eden Prairie, MN) was attached to the margin of the cross-heads for measuring the local strain of the tendon. The specimens were kept moist with normal saline during the entire testing process. The specimen was pre-conditioned with 10 oscillation cycles of 2.5% strain at 10mm/min to minimize the effect of deep freezing [20], and then stretched to 2.5% strain and maintained for 5 minutes. The load required to sustain the length of specimen was recorded at 5 Hz throughout the test. The percentage change in load reflected the load-relaxation property. The specimen was then subject to ultimate tensile failure testing at a strain rate of 500mm/min with data sampling rate of 50Hz [14]. The ultimate failure load (UFL) was defined as the maximum load before failure and the structural stiffness was defined as the gradient of the linear portion of the load-deformation curve.
Statistical Analysis
Statistical analysis was performed using SPSS ver.20 (IBM, USA). Results were presented as means and standard deviations. Intergroup differences in biomechanical results and cell densities were evaluated with one-way ANOVA with post-hoc LSD test. Statistical significance was set at p<0.05.
Results
Histological Analysis
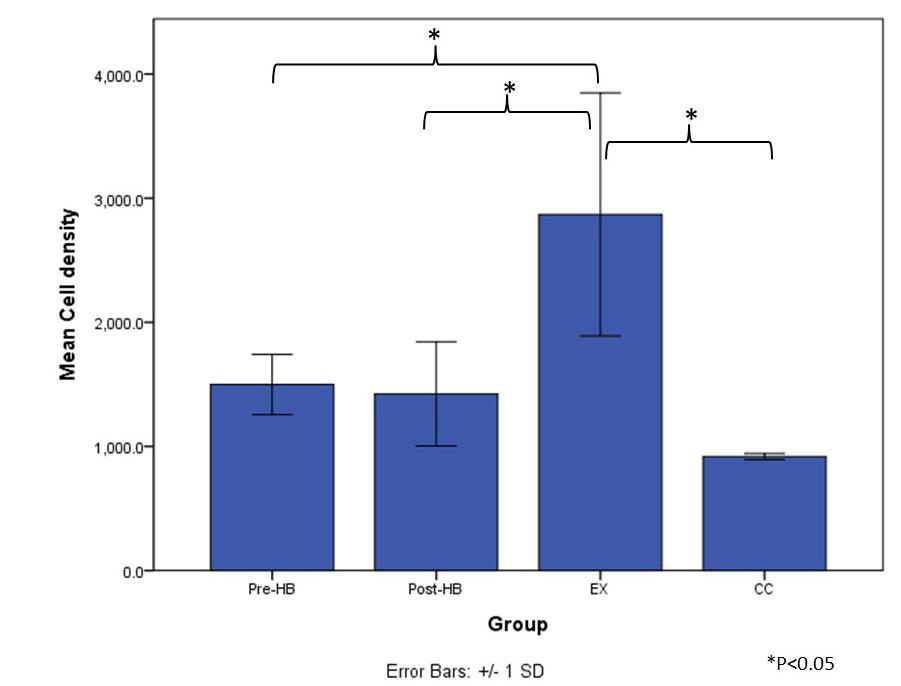
After 8 weeks of bipedal downhill running exercise program, when compared to CC group (Figure 2a), hypercellularity was observed in all tendon specimens of EX group through intra-group variation at the level of cellularity existed (Figure 2d). Besides, mild level of hypercellularity was found in some of the tendon specimens of Pre-HB and Post-HB groups (Figures 2b & 2c). The average density of cell count of EX group (2868.7±979.3 /mm2) was significantly higher than those of CC group (918.2±24.4 /mm2) (p=0.002, post-hoc LSD), Pre-HB (1498.7±243.1 /mm2) (p=0.015, post-hoc LSD) and Post-HB group (1423.1±419.7 /mm2) (p=0.012, post-hoc LSD). However, no difference in cell density was found among CC, Pre-HB and Post-HB (Figure 3).
Figure 2: Histomorphology of Achilles tendons after treatment.
a) Cage control (CC);
b) Exercise with pre-exercise herbal treatment (Pre-HB);
c) Exercise with post-exercise herbal treatment (Post-HB); and
d) Exercise only (EX). Hypercellularity was observed in exercise-only animals.
Biomechanical Testing
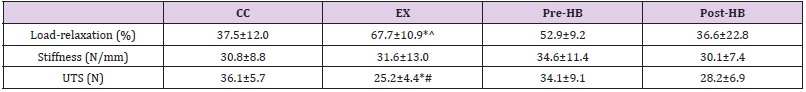
The impact of overuse on load-relaxation was significant that load-relaxation of EX was significantly higher than those of CC (p=0.002, post-hoc LSD) and Post-HB (p=0.002, post-hoc LSD) but not Pre-HB (p=0.097, post-hoc LSD). In addition, the difference in load-relaxation among CC, Pre-HB and Post-HB was not significant. Besides, post-hoc LSD tests revealed no significant difference in structural stiffness among all groups. However, the UFL of EX was found to be significantly lower than both Pre-HB (p=0.034, post-hoc LSD) and CC groups (p=0.011, post-hoc LSD) whereas no difference in UFL existed between Post-HB and EX (p=0.448). Further, there was no significant difference in UFL observed among CC, Pre-HB and Post-HB (Table 1).
Table 1: Biomechanical results.
Note: *p < 0.05 against CC, ^p < 0.05 against Post-HB, #p < 0.05 against Pre-HB.
Discussion
With 8 weeks of forced bipedal downhill treadmill running exercise program, The Achilles tendons of EX group demonstrated features of tendinosis. Repetitive micro-traumas induced by overuse and mechanical overload were proposed to be causative factors for tendinosis [21]. As the degenerative condition progresses, the fibroblasts’ proliferation and collagen fibers disintegration become evident. The collagen fibers become progressively disorganized with tearing at the microscopic level [22]. These changes would adversely affect the viscoelastic properties and mechanical performance of the Achilles tendons [23]. The findings in EX group revealed a significant increase in cell numbers and load-relaxation as well as a significantly lower UFL as compared with CC group. These findings were comparable to the pathological changes of degenerative tendons described in previous reports [17,23,24] which provide further proof that Achilles tendinosis had happened in EX group after the running exercise program.
Histological findings in both Pre-HB and Post-HB groups revealed no difference in cellularity with CC group but they were significantly lower than that of EX group (Figure 3) through mild level of hypercellularity was observed in some of the specimens in the groups. In Post-HB, the load-relaxation was significantly lower than that of EX group (Table 1) suggesting that the application of current herbal medication after forced running period would likely facilitate healing of the degenerative Achilles tendons. Importantly, this highlighted post-injury composite herbal treatment was beneficial to the viscoelastic performance of the tendon. Viscoelasticity affects tendon’s ability to store, translate and dissipate energy as well as to adapt to loading conditions over time [25]. As the foot lands on the ground during gait, deceleration of the body begins, and the tendons and muscles are stretched by the impact forces. As the foot leaves the ground during push-off, elastic recoil from the tendons would convert most of the stored energy back to kinetic and potential energy [26]. Thus, viscoelasticity of tendons would determine the capacity of strain energy stored in tendons as occurs in fast locomotion [27]. A former report demonstrated that altered viscoelastic properties in degenerative Achilles tendons would affect explosive performance in elite athletes and the alterations would influence movement accuracy and energy efficiency in the ankle joint as well [28]. Importantly, if a tendon does not exhibit normal viscoelasticity and becomes too compliant, the capacity for it to store energy as elastic energy would be limited and positional control would be hampered thus increasing the risk of injury [29].
On the other hand, degenerative changes of tendons were reported to be associated with ruptures. For instance, rotator cuff disorders were the most common causes of shoulder disability and common in the middle age or senior population. The occurrence of full-thickness rotator cuff tears appeared to be increased with age [30]. The etiology of rotator cuff tearing was multifactorial and likely a combination of micro-/macro-trauma and age-related degenerative changes such as tendinosis [31]. Thus, preventive measures for tendinosis would not only help preserve structural and functional qualities of the tendons but likely reduce the risk of tendon ruptures. However, investigation on prophylactic efficacy of topically applied composite herbal medication was limited. In current biomechanical results, no significant difference in biomechanical properties was observed between Pre-HB and CC, but the UFL of Pre-HB was significantly higher than that of EX (Table 1). Decreased strength was one of the major biomechanical features of Achilles tendinosis [18,19] Thus, in addition to preserving cellularity of tendons, pre-exercise treatment of current composite herbal medication would help preserve mechanical properties of Achilles tendons as compared to EX group.
This study demonstrated both healing and prophylactic effects of composite traditional Chinese herbal medication on overuseinduced Achilles tendinosis in terms of preserving cellularity and biomechanical properties. However, only cellularity was examined in the current study which may not reflect the entire histological morphology of the tendon specimens. Further analysis on collagen fiber orientation, thickness and occurrence of micro-traumas would provide more comprehensive description of the morphology. On the other hand, the pharmacological mechanism and the mediators for the active ingredients were still unknown. Further investigations on these issues would provide insights for enhancing the efficacy of the current herbal formula.
Conclusion
The herbal formula of Dipsaci Radix (DR), Rhizoma Notoginseng (RN), Flos Carthami (FC) and Rhizoma Rhei (RR) was demonstrated to have both healing and prophylactic potential for overuseinduced Achilles tendinosis in rat model by preserving cellularity and mechanical properties.
For more Articles on: https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.