An Intrinsic Need for Vitamin K2-7 Supplementation: A Narrative Review of K2-7 and Peripheral Neuropathy
Introduction
Since its description as a clotting factor in 1936, vitamin K has received increased attention of the scientific community over the past 80 years, due to its remarkable health benefits. Among its two biologically active forms - K1 (Phylloquinone) and K2 (menaquinone), the latter participates in several physiological functions beyond clotting [1]. Both the forms function through the same process of carboxylation of vitamin K-dependent proteins (VKPDs). The carboxylated VKPDs, through their calcium binding affinity, prevent calcium deposition at ectopic sites and facilitate deposition at appropriate sites, i.e., more in bones and less in soft tissue and vascular walls. Though both Vitamin K1 and K2 have naphthoquinone as a basic structure, their different side chains elicit different pharmacokinetic/pharmacodynamic (PK/PD) properties as well as render differences in their functional sites, e.g., bone and liver [2]. Compared to K1, K2 is readily absorbed, and has a long plasma half-life. Vitamin K2-7 has a longer half-life of 3 days as against 3 hrs for K1 [3,4]. Vitamin K1 primarily facilitates coagulation function whereas K2 is involved in anti-coagulant and non-coagulant functions. Further, K2 has different subtypes referred to as MKn, where n stands for the number of prenyl units varying between 1 to 12. Apart from this difference, Vitamins K1 and K2 also differ in dietary sources. An ample amount of K1 is present in vegetables, fruits, nuts, dairy products and in meat and fish.
However, K2 is largely of microbial origin and hence found in some fermented foods, and in animal products. Vitamin K2 insufficiency is rampant due to its limited dietary source, especially in populations consuming a strictly vegetarian diet. Among the menaquinones’ subtypes – MK-7 i.e., Vitamin K2-7 has been extensively studied for its role in cardiovascular and bone health, neurological and immune function as well as in various disease conditions – diabetes, cancer, osteoporosis, bone fractures, arthritis, kidney diseases, and liver diseases. Over two decades, research focus revolved around 3 major aspects viz.
i) Vitamin K2-7 status in the diet of various populations and its association with the prevalence of a disease
ii) Vitamin K2-7 dietary source and its bioavailability
iii) Supplementation of Vit K2-7 and its benefits in various ailments.
Vitamin K2-7 Health Benefits
Our research group has extensively studied Vitamin K2-7 in all these aspects. We have undertaken extensive research on Vitamin K2-7 experiential effects. We have discovered a wider role for Vitamin K2-7 in energy homeostasis (VO2 max) [5], peripheral neuropathy [6-10] and muscle cramps [11]. Recently, we have reviewed the potential role of K2-7 in preventing and/or reducing COVID-19 morbidity and mortality [12]. We have reported Vitamin K2-7’s pivotal role in mitochondrial ATP generation by acting as a mitochondrial electron transport chain [13]. Our patent describes the uses of vitamin K, its analogues, and derivatives in a diverse array of disease conditions such as improving blood perfusion and ameliorating hypoxia in prevention and treatment of conditions such as chronic venous insufficiency, post thrombotic syndrome, skin conditions related to melanization, hyperpigmentation, paresthesia, oedema, varicose veins, and cramps [14].
Another patent describes the role of vitamin K, its derivatives, and combinations to increase the energy levels in diverse disease states and lifestyle disorders, which are characterized by low energy level due to inadequate VO2 max and PO2 and low availability of ATP molecules [15]. Further, we have reported that Vitamin K2-7 can restore the sympatho-vagal balance and have cardioprotective effect by shortening of QT interval and prolongation of RR interval [16] and also prove useful in increasing high density lipoprotein [17]. The present article gives an overview of the research on vitamin K2-7 and summarizes the extensive clinical work conducted by our research group in peripheral neuropathy. We also provide a mechanistic basis for the beneficial effects of vitamin K2-7 in ameliorating peripheral neuropathy including a putative role for noncoding RNAs such as microRNAs (miRNAs).
Dietary Vitamin K2-7 in the Population and its Association with Health and Disease
The early evidence of association of Vitamin K2-7 with cardiovascular disease came from two major populationbased studies. A study was conducted by Geleijnse, et al. [18] at Rotterdam in around 4800 subjects who were followed for the development of aortic calcification and coronary heart disease over 7-10 years. Based on the dietary intake data, they reported 50% reduction of cardiovascular deaths with those who consumed 45 μg of Vitamin K2-7 and 25 % reduction in cardiovascular mortality with daily intake of 21 to 32 mcg Vitamin K2-7. This dose to disease correlation was soon confirmed by Gast, et al. [19] in the cohort consisting of 16,057 women, which further reported 9% reduction in the risk of cardiac disease at the consumption of every 10 μg MK-7 per day. During this period, Spronk, et al. [20] in 2003 conducted an in vivo study for assessing tissue specific utilization of vitamin K2 and found experimental evidence to show that vascular health improved with K-2. It is now confirmed that K2-7 prevents deposition of calcium in the cardiac arteries, and in turn prevents the arteries from stiffening.
In case of bone health, the study by Knapen, et al. [21] in 2013 showed the role of vitamin K2-7. In this placebo-controlled study in 244 postmenopausal women, low dose K2-7 (180 μg/ day) significantly prevented the age-related decline in Bone Mineral Content (BMC) and Bone Mineral Density (BMD). It also reported improved vitamin K status determined by the circulating uncarboxylated osteocalcin (ucOC) and carboxylated OC (cOC). The association of low vitamin K intake and high circulating ucOC with low bone mass and increased fracture risk was already reported [22-26] and the study by Knapen, et al. [21] gave the first clinical evidence that the supplementation of K2-7 can benefit health.
Alarmingly Low Levels of Vitamin K2-7 Globally
Various prospective population-based studies conducted in the last decade has supported the fact that various populations across the globe have Vitamin K2-7 insufficiency. Serum concentrations of vitamin K2-7 are higher in frequent natto (900 mcg/100 gm) eaters. Natto is a popular breakfast item in Japan (more in Eastern Japan as compared to Western Japan). The study by Kaneki, et al. [27] reports an average serum Vitamin K2-7 concentration of 5.26 ng/ml in Eastern Japanese women (Tokyo), 1.22 ng/ml in the Western Japanese women (Hiroshima) and 0.37 ng/ml in British women (London). It is interesting to note that the ratio of Vitamin K2-7 serum levels in the eastern Japanese women to that of British women is 15:1 which inversely mirrors the fracture rate of 1 in Japanese women to that of 15 in the British women. Results of the deficiency of Vitamin K2-7 in the supplementation through food is clear. Schurgers, et al. [28]. have studied levels of Vitamin K2-7 in many food products globally and found that the Vitamin K2-7 levels are quite negligible in all the food products except natto, a staple food in Eastern Japan which contains almost 998 mcg of Vitamin K2-7 per 100 gm of Natto. The investigators also found small amounts of Vitamin K2-7 in natural cheese. Our recent work also highlights alarmingly low levels of Vitamin K2-7 in serum of healthy as well as diabetic Indian population. The common staple Indian foods tested in this study had undetectable levels of vitamin K2 [29]. This data and the literature projecting poor Vitamin K2 status in the population worldwide and its limited dietary source emphasize the need for its supplementation. We have also demonstrated beneficial effects of Vit. K2-7 in various ailments as diabetes, muscle cramps, neuropathy with various mechanisms (vide supra). The following section summarizes our clinical work in peripheral neuropathy.
Peripheral Neuropathy
Peripheral neuropathy is a condition that results from the altered or disordered transmission of sensory signals from periphery to brain and spinal cord. Hence, by the newer definition given by the International Association for the Study of Pain [30], peripheral neuropathy is a ‘pain induced by a lesion or disease of the somatosensory nervous system. Peripheral neuropathy primarily affects the pain-neuro axis and covers innumerable conditions viz. vitamin deficiency, chemotherapy-induced neuralgia, trigeminal neuralgia, diabetic neuropathy, HIV infection, leprosy, amputation, peripheral nerve injury and stroke. The key symptoms experienced by patients are numbness, cramps, and tingling, burning and electrical-like sensations, and pain resulting from non-painful stimulations such as light and touch. At initial stage, neuropathic pain is the body’s natural response to protect the damaged region, but with chronic condition both frequency and severity increases leading to sleep disturbances, anxiety, depression, thus affecting the overall quality of life.
Neuropathic pain affects 20-26.4% of diabetic patients [31] and 20% of patients with herpes zoster in the United States [32]. Further, 48-74% of patients with low back-related leg pain [33] and 40% of people after surgery suffer from neuropathic pain [34]. In addition, 8.1-17.9% of the Canadian population is affected by neuropathic pain [35]. The symptoms of neuropathy were observed in 26- 31% of South Indian population with diabetes with almost 10.4 million people with diabetes across India suffering from symptoms of neuropathy. Various treatment modalities are available for peripheral neuropathy in vitamin B12 deficiency. Despite these treatment modalities, there is persistence of neuropathy symptoms. Although there is an improvement in the vitamin B12 levels of these patients, they still suffer from neuropathy symptoms. This is known as residual neuropathy. Similarly, despite good glycemic control, diabetic patients continue to experience symptoms of neuropathy again symptomatic of residual neuropathy. Persistence of residual neuropathy symptoms despite treatment makes it an unmet medical need. It was of interest to observe that ten out of 23 patients of vitamin B12 deficiency group in Kulkarni, et al. [6] had residual neuropathic symptoms in-spite of adequate levels of vitamin B12 following vitamin B12 administration. The residual neuropathic symptom score reduced following vitamin K2-7 therapy.
Vitamin K2-7 in Peripheral Neuropathy: Clinical Evidence
We have conducted a set of clinical studies in patients suffering from peripheral neuropathy in a subset of patients having vitamin B12 deficiency and suffering from diabetes &/or hypertension. In addition, an observational study was conducted in a small number of multiple myeloma patients who developed iatrogenic peripheral neuropathy. All the patients were supplemented with 100 μg twice a day Vitamin K2-7 for 8 weeks. The results of these studies are summarized below.
An open labelled study: This was a preliminary observational study in 30 patients of peripheral neuropathy complaining tingling and numbness along with weakness, fatigue and cramps [6]. Seven patients were diabetic and 23 were suffering from megaloblastic anaemia. Despite having good glycaemic control, and adequate serum levels of Vitamin B12 (due to Vitamin B12 supplementation), these patients were suffering from peripheral neuropathy. Twentyfive of them had severe symptoms with Visual Analogue Scale (VAS) score between 8 to 9 and rest had moderate symptoms with VAS score up to 6. Both the groups showed decrease in VAS score when flowed up at 4th week and their VAS score reduced to 2 by 8th week. This study also established tolerability and organ safety of Vitamin K2-7, as all biochemical investigations and organ functions tests were normal at the end of 8 weeks.
An open labelled expanded study: The encouraging results of the first observational study prompted a further study in a large number of patients (n=100) [7]. Patients suffering from peripheral neuropathy diabetes (n=53) or Vit. B12 deficiency (n=47) were included. Patients were followed up for every 15 days up to 8th week of treatment and additional 15 days post-treatment. The study confirmed the finding of the observational study and VAS score remained <2 at the end of 12 weeks. Tingling, pricking sensation and cramps were most common symptoms in both the groups with VAS score 7 to 9 which reduced to <2. The safety of vitamin K2-7 was also evident in this study population.
First randomized placebo-controlled double-blind study: The study was conducted in another set of patients (n=60) with placebo control to confirm that the effect seen in the above study is due to Vitamin K2-7. The study confirmed the earlier findings of reduction in VAS score in both diabetic and Vitamin B-12-deficient patients from 8 to 1.5 which remained unchanged in the placebo group [8].
Second randomized placebo-controlled double-blind study: This study was an extension of the 3rd study above and was conducted in a small number of patients (n=20) with the same clinical protocol [9]. In addition, Vitamin K2-7 levels were measured in all the patients at baseline and at 4th and 8th week. Vitamin K2-7 supplementation showed significant rise in serum levels in the study group, which correlated with the clinical response.
Effect of Vitamin K2-7 in iatrogenic peripheral neuropathy: The above study gave promising results in peripheral neuropathy due to diabetes or Vitamin B12 deficiency. This was followed up by an investigation into another etiology of peripheral neuropathy. An open labelled observational study was conducted in patients with multiple myeloma [10]. Seventeen patients complaining tingling, numbness and burning sensation were selected for the study and were given 100 μg or 350 μg Vitamin K2-7 twice a day till the end of 4th chemotherapy cycle. Assessment was done at the end of each chemotherapy cycle. The study suggested for the first time that vitamin K2-7 ameliorates iatrogenic peripheral neuropathy in multiple myeloma patients.
Vitamin K-2 in Peripheral Neuropathy: Molecular Aspects
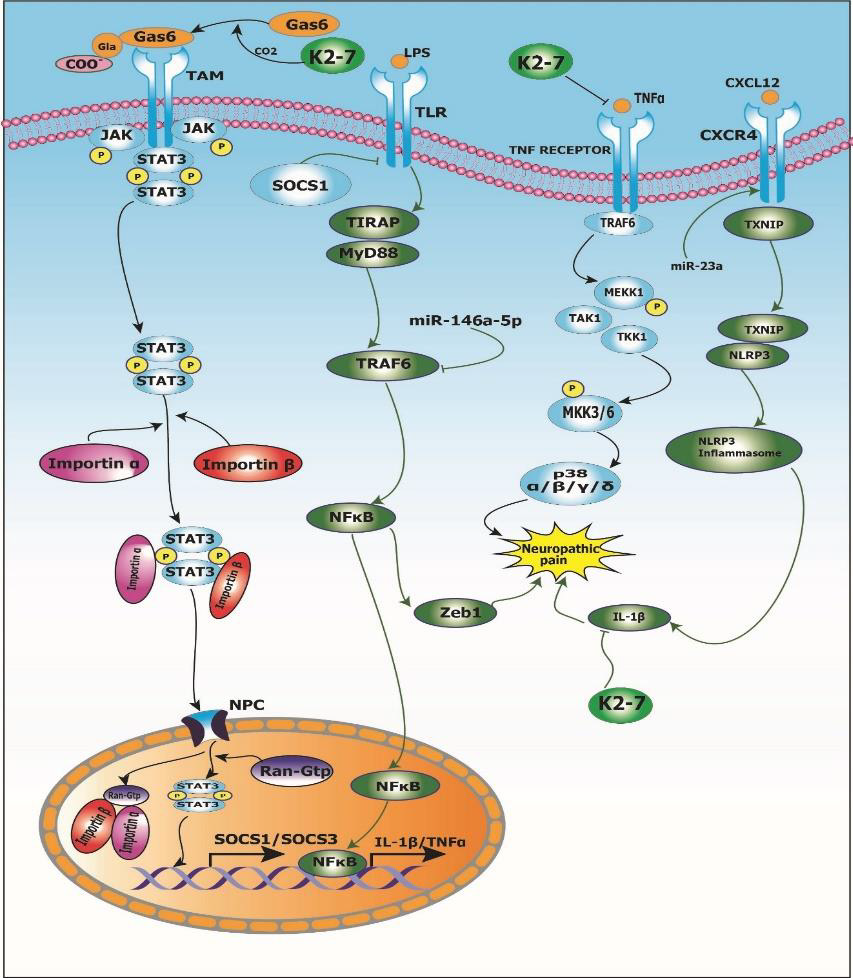
Peripheral neuropathy is a chronic condition characterized by pain, numbness, slow nerve conduction and tingling in the extremities [36]. Several pathophysiological mechanisms of peripheral neuropathy have been described. The beneficial effects of vitamin K2-7 in peripheral neuropathy are mediated via various signal transduction pathways including, but not limited to, Gas6/ TAM signaling, cytokine signaling including TNFα/IL-1β, and chemokine signaling through CXCR4/NLRP3 inflammasome, amongst others. We also propose that vitamin K2-7 may modulate various miRNAs for exerting its ameliorative effects in peripheral neuropathy. Figure 1 represents signaling events in neuropathic pain that are modulated by vitamin K2-7.
Gas6/TAM Signaling Pathway: Schwann cells are the most abundant glial cells in the peripheral nervous system and demyelination of peripheral nerve fibers in this cell is one of the causes of peripheral neuropathy [37]. Demyelination leads to destruction of the structural and molecular features of the nerve fibers, which develops peripheral neuropathy. Vitamin K2 plays a role in the repair and synthesis of myelin in the peripheral nervous system. Vitamin K2 activates growth arrest-specific 6 (Gas6) protein, a vitamin K-dependent protein by carboxylation of Gla residues of Gas6, which is homologous to the anticoagulation factor protein S. Gas6 and protein S bind to form a complex and activate the receptor tyrosine kinase of TAM (Tyro3, Axl, and Mer) family by binding to the laminin like globular domain of Gas6 which leads to increased myelin production and repair after myelin injury. Goudarzi, et al. [38] investigated the role of Gas6 protein in myelination in humans using C57/BL6 mice. It was reported that Gas6/TAM signaling led to oligodendrogenesis as well as increase in expression of myelin basic protein (MBP) via Tyro3 receptor. Also, Gas6 activated STAT3 signaling and downregulation of matrix metalloproteinase-9 (MMP-9) which was indicative of neuroglial cell repair. Therefore, it was concluded that vitamin K-dependent Gas6 protein can be a therapeutic target in various neuropathologies such as neuropathic pain.
Inflammatory Pathways in Peripheral Neuropathy: Proinflammatory cytokines such as interleukin-1beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are upregulated in spinal cord glial cells as well as dorsal root ganglion (DRG) after nerve and tissue damage under conditions like painful neuropathy [39]. Lu, et al. [40] investigated the role of proinflammatory cytokines, viz., TNF-α and IL-1β expression in tumor necrosis factor receptor-associated factors 6 (TRAF6) signaling using adult ICR mice. It was observed that TNF-α led to an increase in NF-ᴋB signaling which resulted in the inflammatory response. Furthermore, TNF-α and IL-1β integration led to increase in the CC-chemokine ligand 2 (CCL2) expression via TRAF6 signaling. Lu, et al. [41] investigated the role of miR-146a- 5p in neuropathic pain using adult ICR mice. It was reported that overexpression of miR-146a-5p led to suppression of TRAF6.
This downregulation of TRAF6 further led to abrogation of TRAF6-mediated NF-κB activation and downstream Zeb1 signaling thus mitigating neuropathic pain. Also, in another study conducted by Pan, et al. [42] to investigate the role of miR-23a in neuropathic pain using partial sciatic nerve ligation (pSNL) model of adult C57BL/6J mice, it was reported that miR-23a upregulation led to an overexpression of chemokine CXC receptor 4 (CXCR4) which led to activation of the NLRP3 inflammasome in neuropathic pain via thioredoxin-interacting protein/NOD-like receptor protein 3 (TXNIP/NLRP3) axis. Taken together, cytokines such as TNF-α/IL- 1β and chemokine CXCR4 are involved in signal transduction events induced in neuropathic conditions. Interestingly, a study conducted by Pan, et al. [43] to study the effect of vitamin K2 menaquinone-7 (MK-7) on the expression of TNF-α and IL-1β using human monocyte-derived macrophages (hMDMs). It was reported that MK-7 led to downregulation of TNF-α and IL-1β gene expression in a dose-dependent manner as well as protein production by healthy hMDMs in vitro. Thus, epistemological evidence indicates that vitamin K2 supplementation may be useful for the treatment and management of painful neuropathy.
MicroRNAs in Neuropathic Pain
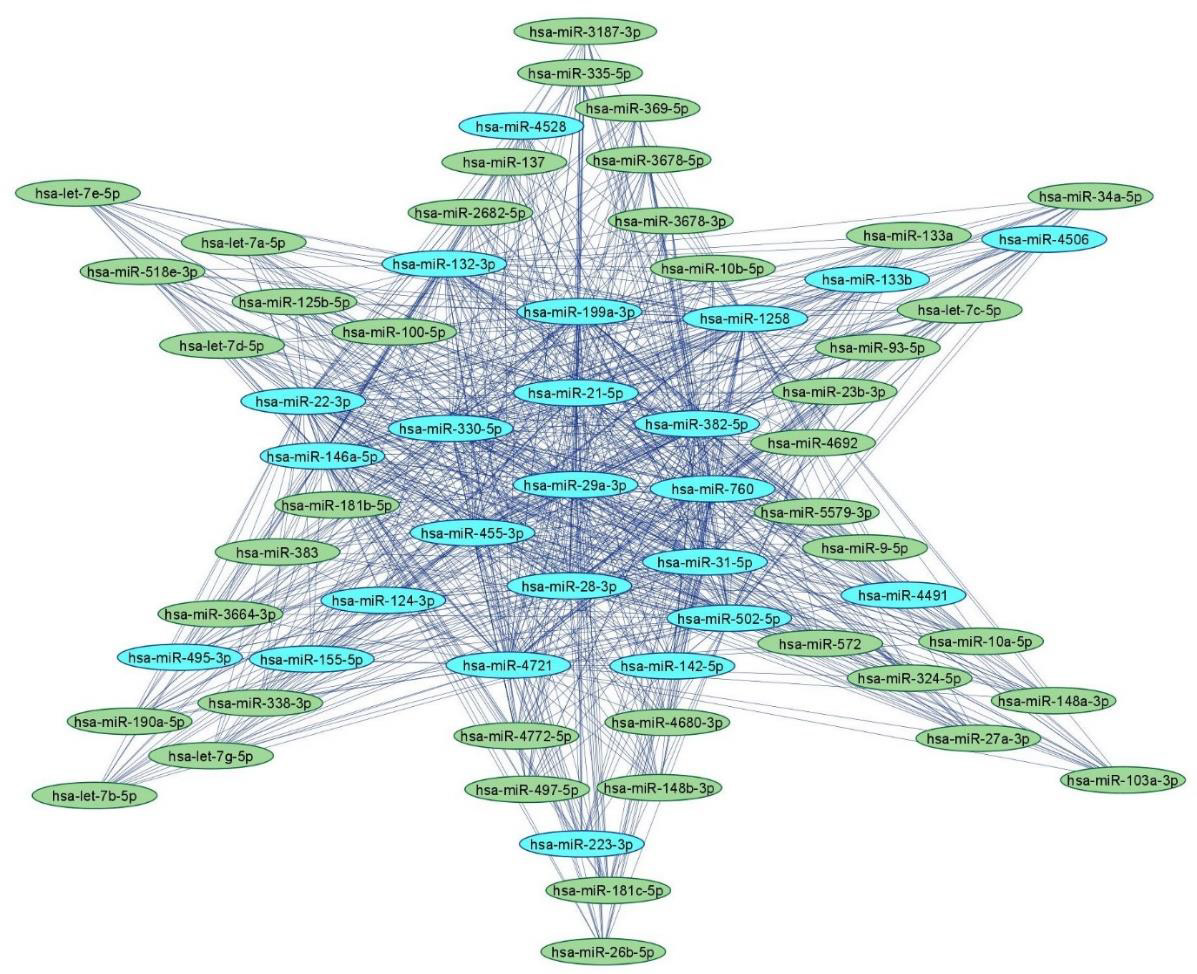
MicroRNAs (miRNAs) are small single-stranded non-coding RNA molecules containing 19–25 nucleotides [44]. miRNAs are ubiquitously present in almost every cellular process by regulating post-transcriptional gene expression and mRNA silencing. miRNAs regulate expression of various genes associated with several diseases [45]. We have previously delineated the roles(s) of miRNAs in the prevention, diagnosis and treatment of various cancers [46- 51]. We have also previously dissected the biological interactome of human and rodent miRNAs implicated in neuropathic pain via bioinformatic analyses [52]. The delineation of the landscape of regulatory miRNA networks in neuropathic pain will enable the discovery of key miRNA/target biomarkers for theragnostic applications in neuropathic pain. Figure 2 depicts an miRNA-miRNA network comprising upregulated and downregulated miRNAs in neuropathic pain based on bioinformatic analyses reported by us earlier [52].
Figure 2: Human miRNA-miRNA network in peripheral neuropathy.
Note: Upregulated (blue) and downregulated (green) human miRNA-miRNA network in neuropathic pain with 65 nodes and 855 edges using miRNAs adapted from [52]. The networks were constructed using Cytoscape 3.9.1.
Upregulated Human microRNAs in Neuropathic Pain: Several microRNAs are either upregulated or downregulated leading to the complications of painful neuropathy. A study conducted by Leinders, et al. [53] showed the effect of hsa-miR-132- 3p in white blood cells (WBCs) as well as sural nerve biopsies of patients with neuropathic pain. It was found that hsa-miR-132-3p was upregulated in both WBCs as well as sural biopsies of patients. Furthermore, another study conducted by Leinders, et al. [54] showed the upregulation of hsa-miR-146a and has-miR-21 in WBCs of patients with painful neuropathies. In addition, hsa-miR-21 was upregulated in the sural nerves in painful neuropathic patients. A study was conducted using TaqMan low density array (TLDA) to measure the level of expression of miRNA in dorsal root ganglion (DSG) of the spinal nerve ligation (SNL) cohort of male Sprague– Dawley rats [55]. Herein, hsa-miR-133b was upregulated in DSG of SNL cohort. Heyn, et al. [56] investigated the role of hsa-miR-124a and hsa-miR-155 in patients with neuropathic pain. It was reported that hsa-miR-124a and hsa-miR-155 was upregulated which led to downregulation of sirtuin1 (SIRT1) mRNA in neuropathic pain patients.
Tramullas, et al. [57] studied the effect of hsa-miR-30c-5p in 25 patients with peripheral ischemia who also showed neuropathic symptoms. It was found that hsa-miR-30c-5p was upregulated in plasma as well as in cerebrospinal fluid (CSF) of patients with painful neuropathy. Xu, et al. [58] studied the SNL model in male Sprague–Dawley rats to study the effects of miRNA in painful neuropathy. It was reported that hsa-miR-22, hsa-miR-133b and hsa-miR-31-5p was upregulated in serum samples of SNL model. Chatterjee, et al. [59] conducted a study in individuals who were diagnosed with arsenic-induced peripheral neuropathy. It was reported that hsa-miR-29a was upregulated in individuals having arsenic induced-peripheral neuropathy. Thus, presence of these miRNAs can be useful prognostic markers in the diagnosis of painful neuropathy.
Downregulated Human miRNAs in Neuropathic Pain: Liu, et al. [60] investigated the role of hsa-miR-101 in sural nerve biopsies of patients with painful neuropathy. It was reported that hsamiR- 101 was downregulated in the plasma and sural nerve biopsies of patients with neuropathic pain. This led to the activation of NF- κB signaling resulting in painful neuropathy. A study investigated the expression of 63 miRNAs in SNL model using TLDA profiling [55]. It was reported that most of the miRNAs were downregulated in SNL model by more than 2 fold including hsa-miR-103, hsa-miR- 181b, hsa-miR-137, hsa-miR-23b, hsa-miR-26b, hsa-miR-148a, hsa-miR-148b, hsa-miR-181c, hsa-miR-125b, hsa-miR-133a, hsamiR- 10a, hsa-miR-10b, hsa-miR-497, hsa-miR-93, hsa-miR-21, hsamiR- 34, hsa-let-7a, hsa-let-7b, hsa-let-c, hsa-let-7d, hsa-let-7d, hsalet- 7e and hsa-let-7g. In another study, Cao, et al. [61] investigated the role of miRNA in skin samples of the patients diagnosed with postherpetic neuralgia (PHN). It was found that hsa-miR-4772-5p was downregulated in the skin samples. Thus, these miRNAs may be potential targets for treating painful neuropathies.
Conclusion
Peripheral neuropathy is a chronic condition that results from diabetes, vitamin B12 deficiency, or chemotherapy-induced neuropathy, and leads to symptoms such as cramps, numbness, and tingling sensations. Management of neuropathic pain is challenging due to its complex and progressive nature and limited utility of therapeutic agents such as gabapentin and pregabalin (Lyrica®). Vitamin K2-7 plays a significant role in alleviating the intensity of peripheral neuropathy symptoms. Clinical studies from our research group strongly validate the utility of Vitamin K2-7 supplementation as a useful intervention to reduce the intensity of neuropathy symptoms. The molecular underpinnings that form the mechanistic basis for the health-beneficial effects mediated by Vitamin K2-7 involve a complex network of miRNA-miRNA interactions and signal transduction cascades that merit further investigation. Taken together, there is a need for supplementation of vitamin K2-7 in the global diet to harness its beneficial effects in the management of peripheral neuropathy of multiple aetiologies.
1. Vitamin K2-7 activates growth arrest-specific 6 (Gas6) protein by carboxylating gamma-carboxyglutamate (Gla) residue present on Gas6; activated Gas6 binds to Tyro3, Axl and Mer (TAM) receptor which leads to dimerization of signal transducer and activator of transcription 3 (STAT3) recruited by Janus kinase (JAK); STAT3 dimer and Importin α/β complex translocate to nucleus through nuclear pore complex (NPC) and stimulates transcription of suppressor of cytokine signaling 1 and 3 (SOCS1/SOCS3). Toll-like receptor (TLR) activates the tumor necrosis factor receptor-associated factor 6/nuclear factor kappa-light-chain-enhancer of activated B cells/zinc finger E-box-binding homeobox 1 (TRAF6/NFκB/Zeb1) pathway that results in neuropathic pain; SOCS1 inhibits TLR and abrogates the TRAF6/NFκB/Zeb1 pathway thus mitigating neuropathic pain. Further, miR-146a-5p downregulates TRAF6 suppressing Zeb1 signaling and alleviating neuropathic pain.
2. Vitamin K2-7 inhibits binding of inflammatory cytokine tumor necrosis factor alpha (TNFα) to its receptor TNFR which abrogates the mitogen-activated protein kinase (MAPK) pathway cascade and ameliorates neuropathic pain. (C) C-X-C motif chemokine ligand 12 (CXCL12) binds to C-X-C chemokine receptor type 4 (CXCR4) and modulates the thioredoxininteracting protein/NLR family pyrin domain containing 3 (TXNIP/NLRP3) inflammasome axis and leads to neuropathic pain via interleukin-1β (IL-1β); vitamin K2-7 inhibits IL-1β and relieves neuropathic pain.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.