Perception Regarding Prevention and Control of Hepatitis Among the Garment Workers of Dhaka
Introduction
Arsenic is the 53rd most abundant element found in nature despite its less abundance in the earth’s crust (0.0001%). Though, arsenic is dangerous for humans in every form, while Arsenic in the +3-oxidation state or arsenite is more harmful as compared to arsenic in the +5-oxidation state or arsenate[1]. Arsenic is widely used in the manufacturing of alloys, mainly with lead and copper, and with gallium in electronic industries as semiconductors. [2,3] Arsenic is toxic to humans in even small quantities. The workers at these factories are at a major risk of developing arsenic toxicities as compared to the normal public, [4,5] but there is a fair chance that non-occupationally exposed persons can get exposed to arsenic [6]. Many forms of arsenic that are more readily absorbed by the human body are more toxic and potentially fatal as compared to the ones that are readily eliminated from the human body [7,8]. Arsenic metabolism in humans plays a very crucial role in causing toxicity. There are various proposed mechanisms of various forms and oxidation states of arsenic [9-15].
Symptoms of acute arsenic poisoning can vary from recurring diarrhea, thickening of the skin, discoloration of the skin, nausea, abnormal heartbeat, numbness in limbs, partial paralysis, and most important and common is occurring of small corns or warts on soles, torso, and palms, whereas chronic arsenic symptoms include darkening skin, constant sore throat and digestive issues [16,17]. Arsenic ingestion in humans can occur from various sources like breathing in air containing arsenic vapors either normally or air coming from a plant, factory, or a mine, smoking any kind of tobacco products, living near an industrialized area or dump/scrap market area, eating any kind of food contaminated with arsenic mainly seafood as it contains low levels of organic arsenic [1,18]. Biological matrices like urine and blood are the most significant for checking the exposure level of arsenic. It takes 3 to 5 days to eliminate about 50% of the total dose ingested through urination, in which dimethylarsonic acid is the major metabolite occurring approximately 60-70% as compared to the other metabolite i.e., monomethylarsonic acid. In cases of acute poisoning, the highest concentration is found in the liver and kidney, as detected by Atomic Absorption Spectrometry (AAS), whereas in cases of chronic poisoning, accumulation of arsenic is seen in kidneys, [19] liver, lungs, and heart in greater extent and smaller amounts in the spleen, muscles, gastrointestinal tract, and nervous system [5,20]. Common methods for quantification of arsenic include Anodic Stripping Voltammetry and Atomic Absorption Spectrophotometry, commonly deployed with Vapor Generator Assembly (VGA) generally known as Vapor generator assembly (VGA) or Flame [21]. Other sensitive techniques are Neutron Activation Analysis (NAA) and Inductively Coupled Plasma (ICP) with Mass Spectrophotometry (MS) or Atomic Emission Spectrometry (AES) [22,23].
AAS-VGA is a very sensitive technique due to its ability to determine trace metals even at very low levels at wavelength below 200 nm. Below 200nm, there are spectral interferences from free radicals of elements in conventional flame AAS [24].
Materials and Methods
Materials
Glassware: A-grade glassware from Borosil Pvt. Ltd. India was used for the experiment. All the glassware were soaked overnight in chromic acid solution and washed with water and finally rinsed with ultrapure water and dried.
Chemicals and Reagents: 1000 mg/L (1000 ppm) Arsenic from Loba Chemicals Pvt. Ltd., 37% Hydrochloric (HCl) of Emparta grade and L-ascorbic acid, Sodium borohydride, Potassium hydroxide of Emsure grade from Merck India were used for the analysis. Ultrapure water from Rions India Pvt. Ltd. was used throughout the experiment. Argon gas of purity 99.99% from Laser Gases Pvt. Ltd., New Delhi, was used throughout the experiment.
Miscellaneous Items: The pipette of a capacity of 1 ml of Corning Company was used. Pipette Tips of 1 ml of Tarson Company were used. The weighing Balance of Igene Labserve was used throughout the experiment.
Instrumentation: Atomic Absorption Spectrophotometer (AAS), Model No. AAS9000 and Hydride Generator (HG), Model No. HG600s from Jiangsu Skyray Instrument Co. Ltd., China was used for the analysis.
Methods
Preparation of Standard Solutions: 1 part per million (ppm) of arsenic stock solution was prepared from 1000 ppm arsenic solution by dilution method. 5 parts per billion (ppb) standard solution was prepared by adding 0.5 milliliters (ml) from 1 ppm arsenic stock solution, 1 gram (g) of thiourea (to make the solution equivalent to 1% thiourea), 1 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid) and 13.5 ml of HCl (to make the solution equivalent to 5% HCl) in a100 ml volumetric flask, shaken well and made up to the mark with ultrapure water. Similarly, 10 ppb, 20 ppb, and 40 ppb were prepared by adding 1 ml, 2ml, and 4 ml from 1 ppm arsenic stock solution in respective 100 ml volumetric flasks by using the above procedure.
Preparation of Standard Blank Solutions: 1 g of thiourea (to make the solution equivalent to 1% thiourea), 1 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid), and 13.5 ml of HCl (to make the solution equivalent to 5% HCl) were taken in 100 ml volumetric flask, shaken well and made up to the mark with ultrapure water.
Preparation of Samples: 3 ml of the urine sample and 3 ml of conc. Nitric acid (HNO3) was mixed in a 10 ml volumetric flask and shaken well, after that it was left overnight. The following next day, the solution was made up to 10 ml by adding ultrapure water to it, which was referred to as stock sample solution.
2 ml of stock sample solution, 0.5 g of thiourea (to make the solution equivalent to 1% thiourea), 0.5 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid), and 6.7 ml of HCl (to make the solution equivalent to 5% HCl) were taken in another 50 ml volumetric flask, shaken well and made up to the mark with ultrapure water.
Preparation of Sample Blank: 3 ml of conc. HNO3 and 3 ml of water were taken in a 10 ml volumetric flask and made up to 10 ml by adding ultrapure water and named it as a stock blank solution. 2 ml of stock sample solution, 0.5 g of thiourea (to make the solution equivalent to 1% thiourea), 0.5 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid), and 6.7 ml of HCl (to make the solution equivalent to 5% HCl) were taken in 50 ml volumetric flask, shaken well and made up to the mark with ultrapure water.
Preparation of Reagents for VGA:
Carrier Reagent: 5% HCl was prepared by dissolving 135.5 ml 37% HCl in a 1-liter volumetric flask and then made up to the volume by adding ultrapure water to it and used as carrier reagent. Reducing Agent: Reducing agent was prepared by dissolving 20 g Sodium borohydride (NaBH4) (to make the solution equivalent to 2% NaBH4) and 5 g Potassium hydroxide (KOH)(to make the solution equivalent to 0.5% KOH) were taken in a 1-liter volumetric flask, shaken well and made up to the volume by adding ultrapure water to it.
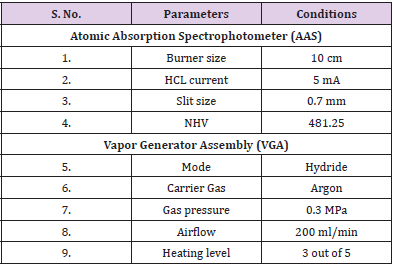
Analytical Procedure: AAS and VGAwere switched ON, Argon gas knob was also opened. The instrument’s software was opened and the arsenic lamp was selected and executed with parameters set according to (Table 1). After that, peak search was started and the absorbance peak of arsenic was checked which must come around 193.7 nm. The instrument is ready to analyze the samples. A new project was selected after that. The operation table was made for 4 standards; 5 ppb, 10 ppb, 20 ppb, and 40 ppb respectively. The concentration was selected as ppb, and method as linear, and then 3 samples were selected and added. Parameters for VGA were set according to (Table 1). Gas pressure and smooth flow were checked, gears of VGA were checked and set accordingly and all the capillary tubes were flushed with ultrapure water before the operation of VGA. After cleaning the capillaries, one capillary was dipped in a carrier solution and the other one was dipped in reducing agent, the last one is dipped in the sample/standard. The pump of VGA was turned on and off according to need, and samples were analyzed. The absorbance of standard blank, standard solutions (5 ppb, 10 ppb, 20 ppb, and 40 ppb) were recorded and the instrument was calibrated. Once calibration was done, a correlation graph was obtained. After calibration, a Sample blank was run, followed by the urine sample. Three consecutive readings of the urine sample were taken to take an average and minimize the error rate of the instrument. All the observations were recorded. After that dilution factor was applied and calculations were done, and the results were established accordingly and printed.
Results
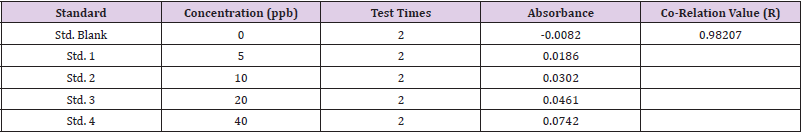
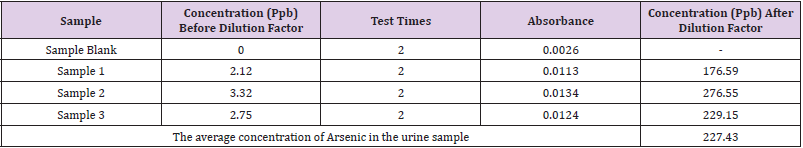
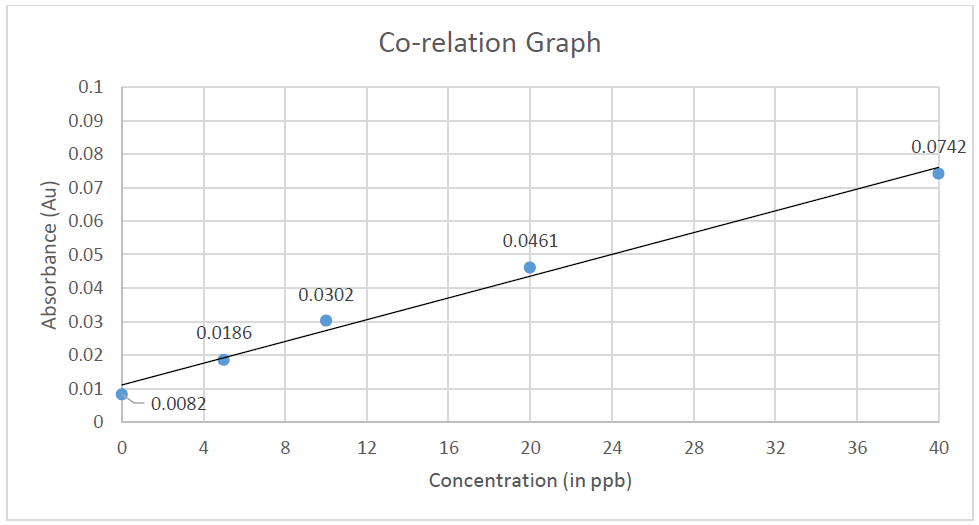
Calibration was done using the arsenic standards prepared. Each standard was tested 2 times and absorbance was recorded (Table 2). A co-relation value (R) of 0.98207 was obtained (Figure 1). After calibrating the instrument, the sample blank was run, following which the sample was run consecutively 3 times to obtain an average reading to minimize possible errors. The sample was tested 2 times during each run and absorbance was recorded. After applying the dilution factor arsenic content came out to be 227.43 ppb in the urine sample. (Table 3)
Discussion
Humans are exposed to arsenic by various exposures routes whether it be occupational, industrial, water, or food consumed. A less common phenomenon is deliberately giving it to an individual in the act of homicide or self-consumption in the act of suicide. For such kinds of cases, a toxicologist/ forensic scientist must be prepared to assess the situation. To save the victim’s life, the forensic scientist must produce accurate results with high precision to treat the poisoning. Common biological fluids and assays for assessing arsenic poisoning (acute or chronic) are generally hair and nails for chronic exposure, and for acute exposure blood and urine are assessed. Atomic Absorption Spectrophotometer combined with Vapor Generator Assembly (AAS-VGA) instrument was used due to its high precision and simplicity. A new method was developed to assess the amount of arsenic in a particular sample, for which the sample needs to be digested before being run on the instrument. After applying the dilution factor and doing calculations, the actual concentration of arsenic in urine was 227.43 ppb or 227.43 ug/L, which is toxic as per WHO protocols and other research studies as well. High acute exposure to any heavy metal can be fatal if not assessed on time. Hence, a cheaper and simpler technique is needed for this purpose.
Arsenic Limit in Urine [10,25-27]
Normal: less than 100 mcg/L (micrograms per liter) M
Moderate: between 100 to 200 mcg/L (micrograms per liter)
Toxic: more than 200 mcg/L (micrograms per liter)
AAS combined with VGA is a much cheaper technique as compared to Inductively Coupled Plasma with Mass Spectroscopy (ICP-MS), Inductively Coupled Plasma with Atomic Emission Spectrometry (ICP-AES), or Inductively Coupled Plasma with Optical emission spectrometry (ICP-OES). With cheap sample preparation and high accuracy in results, this technique still dominates the trace metal analysis section as a whole, else ICP-MS is the gold standard in the section. A highly selective, sensitive, and reproducible AASVGA method has been developed. This method uses a simple and customizable program. The developed method is sensitive enough to support the cleaning validation regulatory studies and can replicate the results up to 1 ppb accuracy. Laboratories with a low budget can use this technique for clinical purposes but in cases of academic/research purposes, where a low level of heavy metal needs to be determined ICP-MS (quadrupole) should be used. Laboratories’ budget will also be a key factor in determining the purchasing, running, and maintenance of the instrument.
Conclusion
Atomic Absorption Spectrophotometer coupled with Hydride Generator was used to analyze a urine sample. Calibration was done from 5 ppb to 40 ppb, the sample was run 3 times, and the average concentration of arsenic was calculated by applying the dilution factor. The concentration of arsenic was 227.43 ppb or 227.43 ug/L. The analyzed result was much higher than the normal limit of arsenic in urine. Hence, the urine sample came out to be within the toxic limit.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.