Elizabethkingia Anopheles Infection after Hyaluronic Acid Injection: A Case Report and Review
Introduction
Elizabethkingia anopheles is an opportunistic pathogen commonly found in soil, fresh water, plants, hospital environments, and contaminated sinks [1]. Sporadic outbreaks of E. anopheles infection have been reported in medical institutions, though point-source outbreaks have also been documented in some countries [2-6]. E. anopheles was isolated and identified from the midgut of the mosquito Anopheles gambiae in Africa in 2011 [7]. E. anopheles infection was first reported in the Central African Republic, where it was reported as neonatal Chryseobacterium meningosepticum infection. Later, 16S rRNA gene sequencing revealed the causative organism as E. anopheles. Several follow-up retrospective tests confirmed that most reported cases of Chryseobacterium meningosepticum infection were actually caused by E. anopheles [4,8]. E. anopheles exhibits broad-spectrum drug resistance. If not promptly identified and treated with appropriate antibiotics, infected patients require more time to recover, and the treatment response may be poor. The mode of transmission of E. anopheles is not completely known [9]. Here, we report a case of E. anopheles infection in the chin after hyaluronic acid injection.
Case Presentation
A 64-year-old woman was diagnosed with cellulitis of the chin in a tertiary care hospital. She had received intravenous antibiotics for 11 days and undergone local incision and drainage (Figure 1A). However, swelling and pain in the mandible did not improve. She also complained of waist soreness and discomfort, pain, and morning stiffness in the metacarpophalangeal joints of both hands. She did not complain of tooth pain or report long-term low-grade afternoon fever, weight loss, night sweats, or history of rheumatoid disease. She had received two injections of hyaluronic acid in the chin at a hospital dedicated for plastic surgery once a year in the past two years. After being admitted to a hospital for the pain and swelling in the chin, she was treated with cefoperazonesulbactam sodium for five days. However, the chin, waist, and metacarpophalangeal joint pain did not resolve. After switching to piperacillin and tazobactam sodium between October 21 and 25, 2020, the symptoms of localized redness, swelling, heat, and pain improved, and the area of infection became darker. From October 26 to 28, 2020, the patient reported worsening of symptoms. Physical examination revealed that the localized redness, swelling, heat, and pain had spread to the skin and soft tissues on both sides (Figure 1B). Leukocyte count and CRP test results suggested that the patient’s infection had a tendency to spread. On October 30, 2020, intravenous moxifloxacin was initiated according to the Microbial DNA Test Report and relevant literature [5,6]. Oral doxycycline was added on November 3, 2020. Bilateral mandibular swelling, heat, pain, waist soreness, and morning stiffness of the metacarpophalangeal joints gradually subsided. The symptoms gradually improved until she was discharged from the hospital on November 9, 2020(Figures 1C-1E).
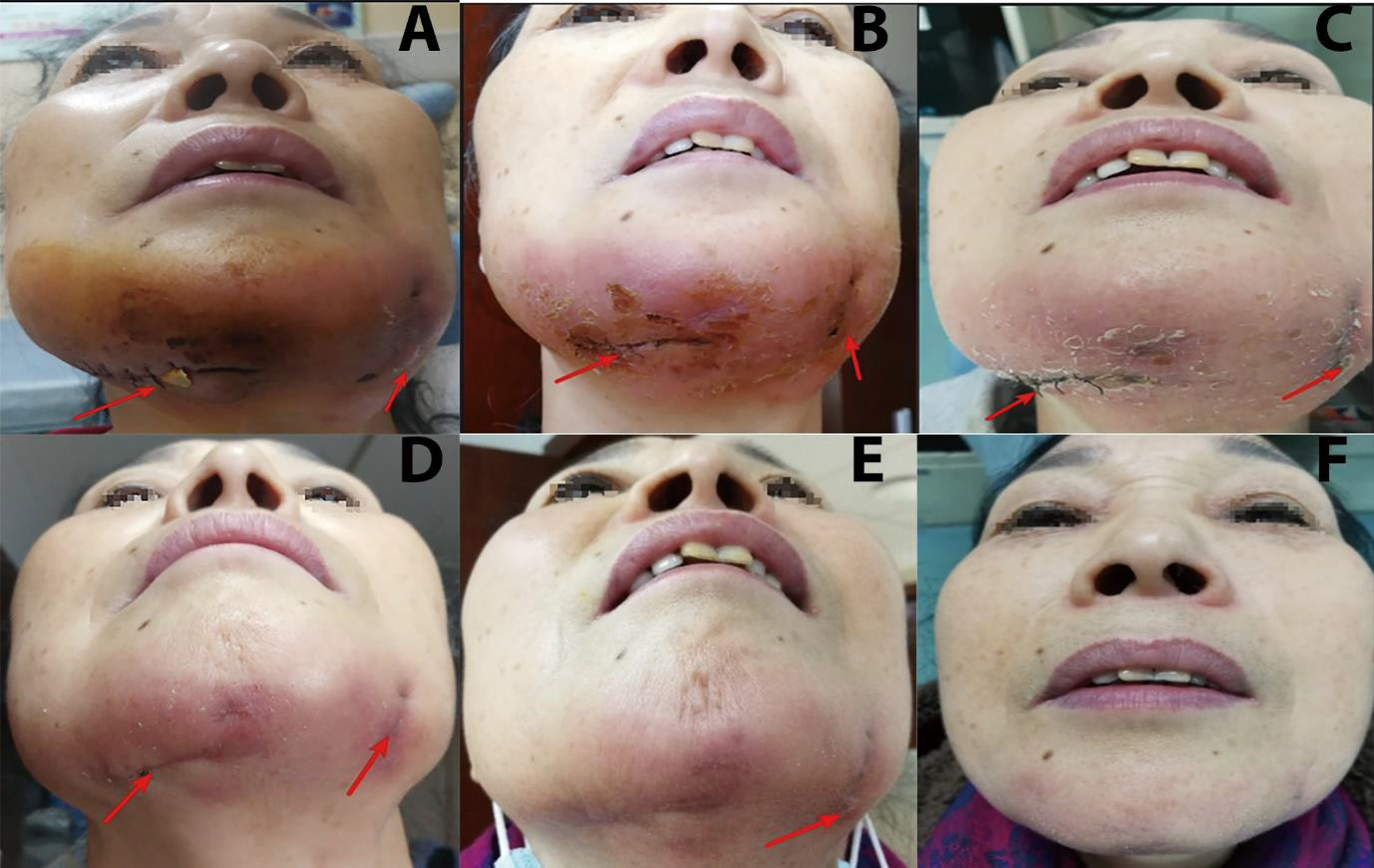
Figure 1: Extraoral photographs of the patient before, during, and after hospitalization.
A. The red arrow shows swelling in the bilateral mandibular region after incision and drainage, but the localized redness, heat, and pain did not show any significant improvement after antibiotic treatment (11 days before admission + 12 days after hospitalization).
B. The symptoms of redness, swelling, heat, and pain aggravated and the lesion showed a tendency to spread. Replacement of the drainage sheet is indicated by the arrow, and samples are taken for routine pathological and microbiological examination.
C. After switching to moxifloxacin, the localized symptoms of redness and swelling disappeared. Healing of the incision is indicated by the arrow.
D. Healing of the incision further improved after suture removal, though one swelling persisted at the left part of the incision as indicated by the arrow.
E. Healing after bilateral incision and drainage following moxifloxacin and doxycycline treatment.
F. The bilateral drainage incisions healed completely after antibiotic withdrawal.
Physical Examination
The mental protuberance and bilateral mandibular region showed pitting edema. Local incision and drainage of the rubber sheets which the tertiary hospital outpatient doctor had put in were observed. A small amount of blood was evident on drainage. No obvious purulent secretions were observed. The patient complained of pain on bending, which was bearable on slow bending. Localized swelling, tenderness, and reduced range of motion were evident in the distal segment of the left little finger. After 4 days of treatment with piperacillin-tazobactam sodium, the localized symptoms were relieved and the area of the swelling decreased, but the pigmentation persisted, tissue was hard, and elasticity was poor. Five days after the administration of piperacillin-tazobactam sodium, localized symptoms aggravated, intensity was greater than that before admission, and a new swelling had developed on the right side of the chin. Localized incision and drainage were performed, and a gray-white purulent discharge was observed. Treatment with moxifloxacin and doxycycline relieved redness, swelling, heat, pain, and symptoms of the waist.
Processing
On October 28, 2020, the patient underwent incision and drainage in the bilateral mandibular region. The wound pus smear was examined under a high-magnification microscope. No gram-negative bacilli were found on the pus swear, and no pathogenic microorganisms were found on the acid-fast stained smears. Meanwhile, the tuberculosis T-cell test, routine pathological examination, and deep-tissue microbial DNA examination were performed. Routine pathological reports suggest local foreign bodies and infections in the chin (Figures 2A-2C). On October 30, 2020, DIAN DIAGNOSTICS of Microbiology Company reported E. anopheles infection. Drug sensitivity and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) tests were not performed because of the limited microbial DNA. According to the literature, we planned to start intravenous 0.4(mg) moxifloxacin Quaque Die (QD) from October 30, 2020. On November 12, 2020, oral doxycycline hydrochloride (0.1 g) QD was started On November 17, 2020 [6,9]. the patient was discharged and advised to continue the medications.
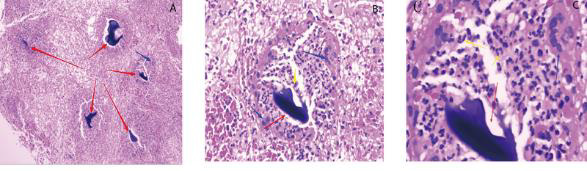
Figure 2: Pathological examination of deep-tissue specimens.
A. The red arrow shows the foreign body, and the blue arrow shows the macrophage reaction (5×10 times the field of view).
B. The red arrow shows the foreign body, the blue arrow shows the macrophage reaction, and the yellow arrow shows the neutrophil reaction (20×10 field of view).
C. Foreign body (40×10 view).
Results
During the 3-month follow-up period from the termination of moxifloxacin and doxycycline treatment, the symptoms in the area of the mental protuberance and bilateral mandibular region subsided, physical examination showed no abnormalities, and the blood leukocyte count, classification of leukocyte, and Antistreptolysin O results were within reference range.
Discussion
In the present report, the patient was diagnosed with a localized E. anopheles infection after injection of hyaluronic acid based on the results of pathological examination, microbiological DNA analysis and microbial infection test results. We did not conduct a follow-up microbiological epidemiological survey because of disputes between patients and hospitals using cosmetic products. It is unknown whether the infection was caused by the injection or use of a contaminated product. As that the patient was initially treated using multiple antibiotics, microbial DNA test results showed that microbial DNA level was low, making accurate diagnosis difficult. Specimens obtained from the lesional site may be contaminated by the normal flora (normal colonizing flora on the skin surface) of the site [10]. Therefore, strict disinfection and aseptic operation are advised to obtain samples from severely infected tissues for accurate diagnosis. Accordingly, microbial DNA and routine pathology sampling were completed simultaneously.
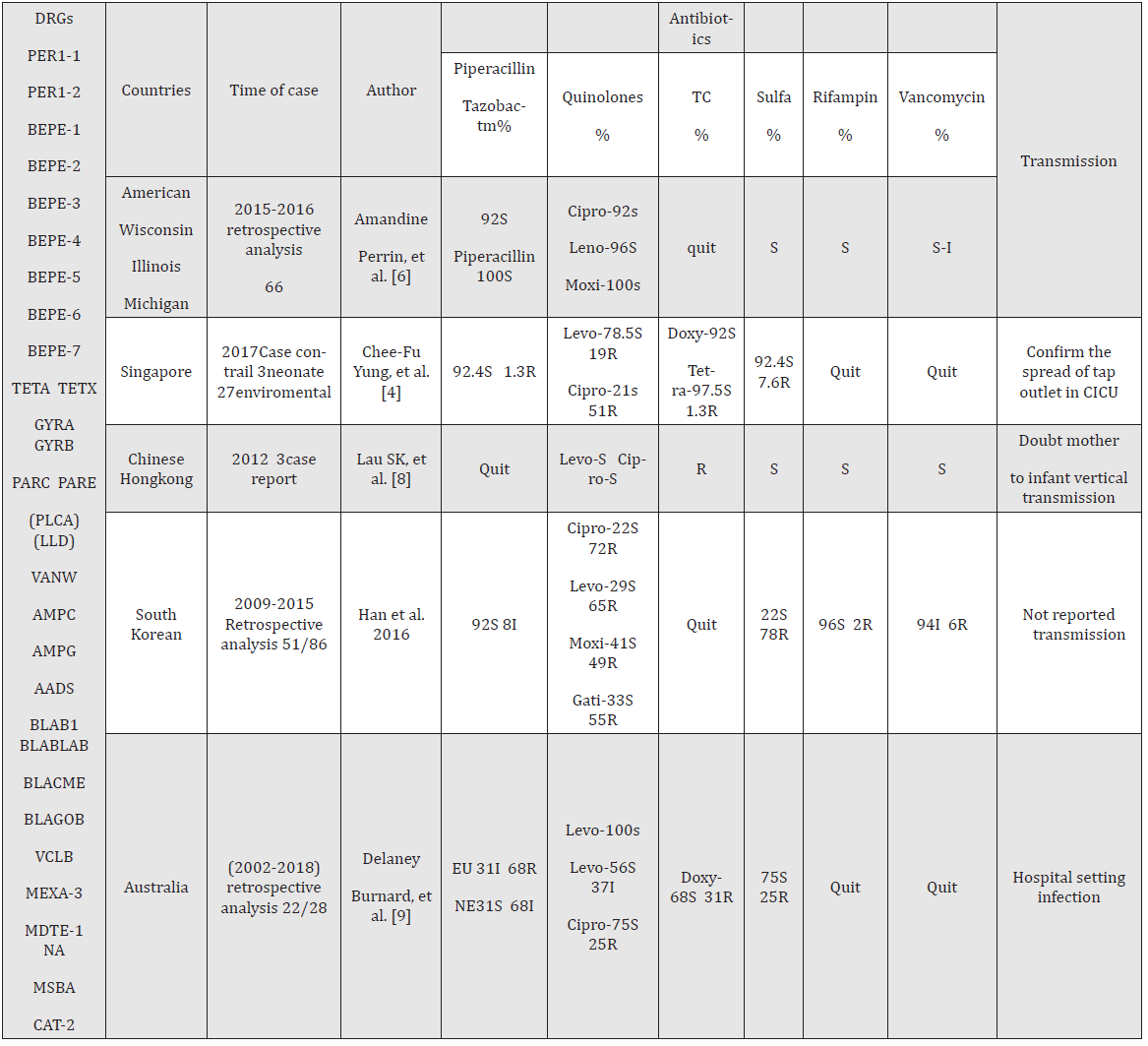
Table 1: The resistance genes were gathered from Mi-Soon Han, et al. [17]; Delaney Burnard, et al.[9]; Susanna [23]; and Amandine Perrin [6]; Drug resistance genes have only been reported by the authors listed above, more in practice, and constantly updated and supplemented. They can be verified using the Comprehensive Antibiotic Resistance Database. Quinolones omitted – floxacin, and tetracyclines omitted – cycline. S: sensitive, R: resistance, I: intermediate sensitivity, NE: non-Enterobacteriaceae, EU: EUCASR, DRG: drug-resistance genes reported in the literature. The numerator represents Elizabethkingia anopheles and the denominator represent the genus Elizabethkingia. The drug sensitivity test included the minimum inhibitory concentration (MIC) and the paper method. Many antibiotics have no breakpoint presently; the effectiveness could be interpreted in the experiment; only the most commonly used antibiotics for the treatment of Elizabethkingia anopheles.
The genus Elizabethkingia includes non-fermenting, non-sporing, non-motile, gram-negative aerobic bacilli. On discovery, CDC ((US Centers for Disease Control and Prevention) Group IIa was assigned to Flavobacterium, which was later reclassified as Chryseobacterium in 1994[11]. In 2005, it was reclassified into a new genus, Elizabethkingia, based on the genotypes and phylogenetic characteristics. The genus contains three medically important species, Elizabethkingia meningoseptica, Elizabethkingia anopheles, and Elizabethkingia miricola [12]. They cause clinical infections to spread mainly by mosquitoes of the genus Anopheles, which induce sepsis in immunocompromised patients and meningitis in neonates [8]. The clinical differential diagnosis of microbial infections is difficult because microbial gene phenotypes are very similar. Genome identification show that the nucleotide sequences of these three microorganisms have extremely high homology (> 98%) [6,8,9]. MALDI-TOF MS, 16S rRNA sequencing, DNA-DNA hybridization, and whole-genome sequencing (WGS) can be used to identify the microorganisms [13]. Owing to the rigid requirements of Food and Drug Adminstration (FDA) approval, strict laboratory requirements, and inadequate understanding of the constitution of E. anopheles, these microbial detection techniques are not commonly performed in ordinary laboratories to differential diagnosis of E.anopheles. Therefore, relevant microbial databases are not updated continually, rendering the diagnosis of their infections difficulty [14].
anopheles infections have been reported sporadically in the United States [6], Central African Republic [15], South Korea [16] Singapore [4], China [17], Chinese Taipei [5,18], and Chinese Hong Kong [19]. Moreover, the number of reports has gradually increased in recent years. Notably, E. anopheles inherently possesses almost all antibiotic-resistant nucleotide sequences (Table 1). However, genes for these hydrolytic antibiotic enzymes do not cause complete resistance in clinical practice. For example, vancomycin, quinones, rifampicin, and aminoglycosides have been reported as effective in clinical infections [14,20,21]. The choice of empirical treatment requires a complex antibiotic selection process involving a combination of literature reports and clinical observations. Patients with deep tissue or organ infections have higher mortality rates [9,22,23]. In the present case, intravenous moxifloxacin was more effective than oral administration in improving symptoms, though some reports indicate that oral antibiotics can effectively treat the infection [24].
The high phylogenetic diversity and temporal and geographic dynamics of E. anopheles render it difficult to develop an ideal treatment regimen. This means that antibiotic resistance may vary according to the species and the region and time of bacterial isolation. Clinically significant differences in the results of drug susceptibility tests for strains in the same region, different hospitals, and different years could be evident [4,6,8,9,17,25]. The common empirical anti-infective program is to choose from the following antibiotics: quinolones, ciprofloxacin, moxifloxacin, tetracycline, minocycline, doxycycline, trimethoprim sulfamethoxazole, rifampin, piperacillin-tazobactam, cefoperazone-sulbactam, and vancomycin, used alone or in combination [8,14,16-18]. However, drug resistance gene tests show that E. anopheles contains the genes for resistance to all these antibiotics. Consequently, it is moderately sensitive or resistant to these antibiotics, which increases the difficulty of antibiotic selection [14, 19]. Identifying the microorganism and selecting antibiotics based on drug sensitivity tests are the most reliable methods for shortening the duration of hospital stay and treatment [9,26].
The transmission route of Elizabethkingia has not been sufficiently investigated. The relationship between the species within the flora and global strain relatedness has not been defined because of continuous evolution of microorganisms [9]. An indwelling catheter in the patient's body has been reported as the primary risk factor for infection. The catheter should be removed during treatment [1,2,8,16]. The infection can spread through contact with contaminated sink taps during hand washing [3]. From 2012 to 2013, Wisconsin, USA, exhibited the largest point-source outbreak since the first report of E. anopheles infection. A CDC investigation confirmed exposure within the health care system prior to the detection of Elizabethkingia spp., of which 2 cases of infection were highly suspected to be related to indwelling catheters in the body, while the rest were not found to be clearly transmitted [6,22]. Water sources within the hospital environment were reported as the main routes of infection in England and Australia, and the concept of water-borne transmission was proposed [2,9]. A maternal amniocentesis/chorial infection has also been proposed [8]. E. anopheles infections have been reported after the consumption of freshwater fish [21,27]. Most literature reports are nosocomial or community-acquired infections. Cultures of various body fluids can test positive for E. anopheles, including blood, lower respiratory tract sputum, cerebrospinal fluid, pleural effusion, abdominal effusion, and synovial fluid. The transmission of this bacterium has not been fully investigated. Although E. anopheles was isolated from the midgut of the mosquito Anopheles gambiae in the Central African Republic, current clinical, environmental, and genetic analysis evidence does not support the theory of mosquito-borne infection [2,6,16,21]. The route of transmission among the population is not determined [24]. This is the first report of E. anopheles infection caused by local injection of medical products.
The clinical features of E. anopheles and other Elizabethkingia infections are not well documented [5]. The risk factors and clinical characteristics of Elizabethkingia infection in patients of South Korea have been reported [16]. Advanced age, malignancy, chemotherapy, residents of nursing homes, neonatal patients, organ transplantation, underlying diseases, indwelling central catheters, exposures within the health care system, hypoalbuminemia, intensive care unit admission, and continued use of carbapenems are risk factors for E. anopheles infection. In this report, the levels of Antistreptolysin O (ASO) were positively correlated with the efficacy of antibiotics. No related adverse reactions have been reported for hyaluronic acid injection products [28].
Conclusion
E. anopheles is a broad-spectrum antibiotic-resistant bacterium. Empirical treatment regimens involve single or combined administration of antibiotic. The choice of antibiotic changes with time and region vary attributed to the continuous evolution of microorganisms. We report the first case of E. anopheles infection after hyaluronic acid injection. Treatment with intravenous moxifloxacin showed better results than oral moxifloxacin. The effects of these treatments were positively correlated with ASO levels. Further studies are needed to determine whether ASO level can be used as an auxiliary diagnostic marker.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.