Trifluoroacetic Acid-Promoted Friedel-Crafts Aromatic Alkylation with Benzyl Alcohols
IntroductionFollowing the 1945 report by Newman on the utility of Trifluoroacetic Acid (TFA) as a condensing agent for the acylation of anisole with acetic anhydride [1], TFA has found enormous utility in organic synthesis [1-3]. For example, TFA is useful in protecting group removal, as a solvent for radical and polymer processes, oxidations, reductions, and other reactions [2]. Some years ago, we described the reduction of diarylmethanols to the corresponding diarylmethanes using the novel combination of NaBH4 and trifluoroacetic acid [4]. During this study we observed the unexpected side reactions shown in Scheme 1, which obviously involve the TFA-promoted self-condensation of the initial reduction products 4, 6, and 8 with the carbocations derived from alcohols 1, 2, and 3 to give 5, 7, and 9, respectively. Although serendipitous, given hindsight this alkylation is not surprising given that TFA is strongly ionizing and poorly nucleophilic, and hence capable of stabilizing, in particular, benzylic carbocations.
Scheme 1: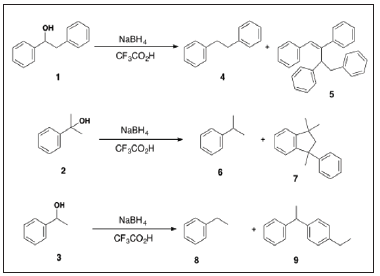 Scheme 2:
Scheme 2: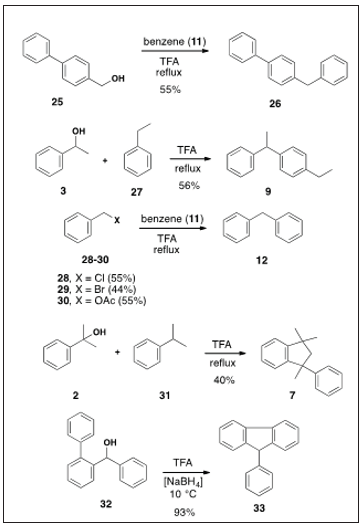 Despite the intervening years, this simple TFA-promoted Friedel-Crafts alkylation of benzylic alcohols has not been pursued. We now describe this alkylation as a simple alternative to the nuTrifluoroacetic merous other methods that have been reported for the benzylic alkylation of arenes with benzyl alcohols (e.g., HF [5], TsOH [6], Nafion-H [7] other cation-exchange resins [8], Sc(OTf)3 [9-11] NiOPO4·3H2 O [12,13], La(OTf)3 O [10], Yb(OTf)3 [10,11] TfOH [10], Hf(OTf)4 [10], rare earth (III) (OSO2C8F17)3 [14] zeolites [15-17], IrCl3·4H2O [18], Ir-Sn complex [19,20], 12-tungstophosphoric acid [21], I2 [22] IrCp* complexes [23], MoO6 [24], NaHSO4/SiO2 [25], Ph3P(OTf)2 [26], Fe(III)-porphyrin [27] BF3-H2O [28], Hf0.5[TEAPS] PW12O40 [29], choline chloride-(TfOH)2 O [30], and B(C6F2)3 [31]. Our method is eco-friendly as the only by-product is water and the TFA can be recycled. A summary of our results is depicted in Table 1. The reactions are not optimized. For example, in one case a 10-fold excess of TFA afforded a 93% yield of 17. In addition, we observed the benzylic alcohol and related alkylation shown in Scheme 2. Interestingly, an attempt to ambush the carbocation from alcohol 32 with NaBH4 [4] was unsuccessful and gave only 9-phenylfluorene [32].
Despite the intervening years, this simple TFA-promoted Friedel-Crafts alkylation of benzylic alcohols has not been pursued. We now describe this alkylation as a simple alternative to the nuTrifluoroacetic merous other methods that have been reported for the benzylic alkylation of arenes with benzyl alcohols (e.g., HF [5], TsOH [6], Nafion-H [7] other cation-exchange resins [8], Sc(OTf)3 [9-11] NiOPO4·3H2 O [12,13], La(OTf)3 O [10], Yb(OTf)3 [10,11] TfOH [10], Hf(OTf)4 [10], rare earth (III) (OSO2C8F17)3 [14] zeolites [15-17], IrCl3·4H2O [18], Ir-Sn complex [19,20], 12-tungstophosphoric acid [21], I2 [22] IrCp* complexes [23], MoO6 [24], NaHSO4/SiO2 [25], Ph3P(OTf)2 [26], Fe(III)-porphyrin [27] BF3-H2O [28], Hf0.5[TEAPS] PW12O40 [29], choline chloride-(TfOH)2 O [30], and B(C6F2)3 [31]. Our method is eco-friendly as the only by-product is water and the TFA can be recycled. A summary of our results is depicted in Table 1. The reactions are not optimized. For example, in one case a 10-fold excess of TFA afforded a 93% yield of 17. In addition, we observed the benzylic alcohol and related alkylation shown in Scheme 2. Interestingly, an attempt to ambush the carbocation from alcohol 32 with NaBH4 [4] was unsuccessful and gave only 9-phenylfluorene [32].
Table 1: TFA-Promoted arene alkylation with benzyl alcohol (10)a.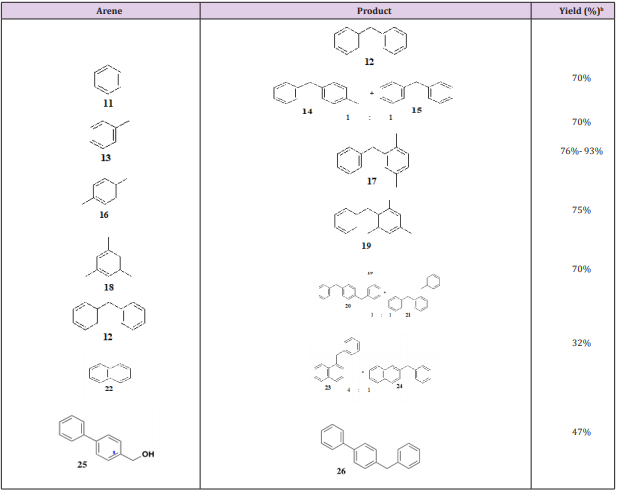 Note: A: Reactions were conducted with a 5-fold excess of arene and TFA as solvent at reflux; B: Yield of distilled product based on benzyl alcohol 10 or 25; C: A ten-fold excess of TFA and 2 grams of benzyl alcohol (10); D: Equimolar amounts of naphthalene (22) and benzyl alcohol (22); E: Yield of crude product.
Note: A: Reactions were conducted with a 5-fold excess of arene and TFA as solvent at reflux; B: Yield of distilled product based on benzyl alcohol 10 or 25; C: A ten-fold excess of TFA and 2 grams of benzyl alcohol (10); D: Equimolar amounts of naphthalene (22) and benzyl alcohol (22); E: Yield of crude product.
To minimize the concentration of TFA, we diluted it with acetic acid (in a ratio of 1:10), but these alkylation conditions of benzene (11) with benzyl alcohol (10) only yielded benzyl acetate, consistent with the enhanced nucleophilicity of acetic acid vis-à-vis TFA. Given the propensity of TFA to promote rearrangements [32,33] in a control experiment we heated 1,4-dbenzylbenzene (20) in TFA for 12 h, but it was recovered unchanged. In the alkylations of toluene (13), biphenyl (12), and naphthalene (22) (Table 1) the expected isomeric mixtures were obtained, and the ratios were estimated by proton NMR integration. In summary, we have described a simple trifluoroacetic-promoted alkylation of aromatic hydrocarbons with benzyl alcohols. The reaction is exceptionally simple, the yields are satisfactory, the byproduct is water, and the TFA can be recycled. Neat TFA enjoys an ideal blend of ionizing power and low nucleophilicity to fulfill a successful arene alkylation of benzylic alcohols.
Experimental SectionThin-layer chromatography was performed using oven-dried Silica gel G plates; benzene was used as a solvent system unless specified otherwise. Spots were observed under ultraviolet light (UVS-11), measuring Rfs from the top of the spot. Gas chromatography analysis was done on a Varian 2800 GC instrument, using CH2Cl2 or ether as a solvent; T = 150 °C unless otherwise indicated. Infrared spectra were recorded with neat films on NaCl plates for liquids, and chloroform solution cells for solids, recorded on a Perkin-Elmer 257 or 137 spectrometers. Polystyrene film was used for calibration. Nuclear magnetic resonance spectra were recorded on a Hitachi Perkin-Elmer R-24 spectrometer, using 5% TMS in CDCl3 as a solvent. Melting points were taken in capillary tubes using a Thomas-Hoover melting point apparatus; they were uncorrected.
General Procedure for the Alkylation of Arenes with Benzyl AlcoholTo a stirred solution of distilled trifluoroacetic acid (10 ml) and arene (0.09 mol) under nitrogen at rt was added a mixture of benzyl alcohol (1.00 g, 0.00926 mol) and arene (0.02 mol). The reaction was heated to reflux for 17 h. Following cooling to rt the mixture was initially concentrated by rotary evaporation (to recover TFA and unreacted arene), and then distilled under vacuum to isolate the diary methane product. In some cases column chromatography was employed.
Characterization Data for CompoundsDiphenylmethane (12): Colorless oil; Yield 64%; bp 88 °C/0.05 Torr (Lit [34], bp 165 °C/24 Torr), which slowly solidified (Lit [34], mp 24.5 °C); IR (film) 3110-–3050, 740, 695 cm-1 (Lit [35] IR (film) 3062, 733, 699 cm-1); NMR (CDCl3) δ 7.5-6.8 (m, 10H), 4.0 (s, 2H) (Lit [35], NMR (CDCl3) δ 7.31-7.17 (m, 10H), 3.98 (s, 2H). GC showed a single peak. This material was identical to an authentic sample [4].
1-Benzyl-4-Methylbenzene (14) and 1-Benzyl-2- Methylbenzene (15): Colorless oil (mixture); Yield 78%; bp 69 °C/0.05 Torr (Lit [7] bp 120–122 °C/3 Torr); IR (film) 3110-2940, 790, 745, 725, 695 cm-1 (Lit [22] IR (film) 3063, 3025, 789, 743, 724, 697 cm-1); NMR (CDCl3) δ 7.3-6.8 (m, 18H), 3.7 (s, 2H), 3.6 (s, 2H), 2.2 (s, 3H), 2.0 (s, 3H) (in a ~1:1 ratio) (Lit [22], (for 14) NMR (CDCl3) δ 7.29-7.08 (m, 9H), 3.93 (s, 2H), 2.30 (s, 3H); GC showed a single peak. Olah was unable to separate 14 and 15 by gas chromatography [36].
2-Benzyl-1,4-Dimethylbenzene (17): Colorless oil; Yield 72%; bp 100 °C/0.05 Torr (Lit [37], bp 115 °C/4 Torr); IR (film) 3030-2900, 890, 730, 690 cm-1 (Lit [22], IR (film) 3025, 2930, 808, 725, 696 cm-1); NMR (CDCl3) δ 7.4-6.9 (m, 8H), 3.8 (s, 2H), 2.3 (s, 3H), 2.1 (s, 3H) (Lit [37], NMR (CDCl3) δ 7.16-6.80 (m, 8H), 3.87 (s, 2H), 2.22 (s, 3H), 2.12 (s, 3H); GC showed one peak.
2-Benzyl-1,3,5-Trimethylbenzene (19): Colorless oil; Yield 73%; bp 105 °C/0.05 Torr (Lit [7], bp 142-144 °C/5 Torr); IR (film) 3015–2880, 850, 725, 695 cm-1 (Lit [35], IR (film) 3020, 2922, 852, 727 cm-1); NMR (CDCl3) δ 7.2-6.8 (m, 7H), 4.0 (s, 2H), 2.4 (s, 3H), 2.2 (s, 6H) (Lit [18], NMR (CDCl3) δ 7.24-7.11 (m, 5H), 6.88 (s, 2H), 4.01 (s, 2H), 2.28 (s, 3H), 2.20 (s, 6H); GC showed one peak. This material was identical to a known sample [4].
1,4-Dibenzylbenzene (20) and 1,2-Dibenzylbenzene (21): White solid (mixture from EtOH); Yield 68%; mp 58–64 °C (Lit [38], for 20, mp 85-86 °C; for 21, mp 78 °C); NMR (CDCl3) δ 7.7-7.0 (m, 28H), 4.45 (s, 4H), 3.90 (s, 4H); the latter peaks in a 3:1 ratio; (Lit [39], (for 20) NMR (CDCl3) δ 7.30 (m, 4H), 7.23 (m, 6H), 7.16 (m, 4H), 3.99 (s, 4H); GC also showed two peaks in a ratio of 3:1. The major isomer (20) agreed by comparison with an authentic sample as prepared below.
1,4-Dibenzylbenzene (20): To a rt mixture of 1,4-diformylbenzene (Aldrich) (10.0 g, 0.0746 mol) in 95% EtOH (530 ml) and water (150 ml) was added NaBH4 (10.0 g, 0.26 mol) over 1 h with stirring. After stirring for 3 h at rt, 6N HCl was added (100 ml). Concentration in vacuo to a volume of 200 ml induced formation of a white solid. Recrystallization from Et2O afforded colorless needles of 1,4-bis(hydroxymethyl)benzene, mp 119-120 °C (Lit [40], mp 117.5-118.5 °C). To a solution of TFA (15 ml) and benzene (25 ml) at rt was added the above diol (1.00 g, 0.00725 mol) in portions over 30 min. The mixture was refluxed for 24 h. The usual workup afforded an oil (2.23 g) that solidified. Crystallization from EtOH gave colorless crystals (0.439 g in two crops) of 20, mp 84-85 °C (Lit [38], mp 85-86 °C). This material was identical to the major isomer 20 as prepared above from diphenylmethane.
1-Benzylnaphthalene (23) and 2-Benzylnaphthalene (24): Colorless oil (mixture); Yield 25%; bp 80 °C/0.25 Torr (Lit [41] 217-230 °C/20 Torr); NMR (CDCl3) δ 8.1-6.9 (m, 15H), 4.4 (s, 2H), 4.1 (s, 2H), the latter peaks in a ratio of 4:1 (23:24); Lit [41], NMR (CDCl3) for 24, δ 7.7-7.0 (m, 7H), 7.08 (s, 5H), 3.92 (s, 2H); Lit [4] (for 23) mp 57.5-59 °C. The major isomer (23) was identical to authentic material by TLC and NMR [4]. 4-Benzylbiphenyl (26). Solid; Yield 47%; mp 84-92 °C after crystallization from Et2O (Lit [42], mp 85 °C; Lit [4] mp 86.5-87 °C); NMR (CDCl3) δ 7.6-6.8 (m, 14H), 4.0 (s, 2H). This material was identical to authentic material [4].
1,1,3-Trimethyl-3-Phenylindane (7): To a solution of TFA (25 ml) and cumene (31) (30 ml, 26 g, 0.22 mol) at rt was added alcohol 2 (2.00 g, 0.0147 mol) over 1 h. The mixture was stirred at 30 °C for 40 h. The usual workup and distillation of the resulting oil gave 7 (1.40 g, 40%), bp 95-97 °C/0.35-0.4 Torr, Lit [43], bp 134-140 °C/10 Torr) (94% by GC), which crystallized on standing, mp 53-58 °C (Lit [43], mp 51-52 °C); NMR (CDCl3) δ 7.3-6.9 (br s, 9H), 2.4 (d, 1H), 2.2 (d, 1H), 1.7 (s, 3H), 1.4 (s, 3H), 1.1 (s, 3H); (Lit [43] NMR (CCl4) δ 7.07-7.03 (m, 9H), 2.41 (d, 1H, AB), 2.15 (d, 1H, AB), 1.63 (s, 3H), 1.30 (s, 3H), 1.01 (s, 3H)). This material was identical to an authentic sample [4]. 9-Phenylfluorene (33). To a mixture of TFA (4 ml) in CH2Cl2 (3 ml) at 10 °C under N2 was added 1 pellet of NaBH4 (0.25 g, 0.0066 mol) followed by known alcohol 3244 (0.250 g, 0.00096 mol). The usual workup gave 9-phenylfluorene (33) (0.217 g, 93%), mp 146-147 °C (Lit [45], mp 147 °C).
Comparison of Outcome Between Proximal Femoral Plate and Cephalomedullary Nail for Intertrochanteric Femur Fracture Fixation in Dr. Sardjito General Hospital-https://biomedres01.blogspot.com/2020/10/comparison-of-outcome-between-proximal.htmlMore BJSTR Articles : https://biomedres01.blogspot.com
 Scheme 2:
Scheme 2: Despite the intervening years, this simple TFA-promoted Friedel-Crafts alkylation of benzylic alcohols has not been pursued. We now describe this alkylation as a simple alternative to the nuTrifluoroacetic merous other methods that have been reported for the benzylic alkylation of arenes with benzyl alcohols (e.g., HF [5], TsOH [6], Nafion-H [7] other cation-exchange resins [8], Sc(OTf)3 [9-11] NiOPO4·3H2 O [12,13], La(OTf)3 O [10], Yb(OTf)3 [10,11] TfOH [10], Hf(OTf)4 [10], rare earth (III) (OSO2C8F17)3 [14] zeolites [15-17], IrCl3·4H2O [18], Ir-Sn complex [19,20], 12-tungstophosphoric acid [21], I2 [22] IrCp* complexes [23], MoO6 [24], NaHSO4/SiO2 [25], Ph3P(OTf)2 [26], Fe(III)-porphyrin [27] BF3-H2O [28], Hf0.5[TEAPS] PW12O40 [29], choline chloride-(TfOH)2 O [30], and B(C6F2)3 [31]. Our method is eco-friendly as the only by-product is water and the TFA can be recycled. A summary of our results is depicted in Table 1. The reactions are not optimized. For example, in one case a 10-fold excess of TFA afforded a 93% yield of 17. In addition, we observed the benzylic alcohol and related alkylation shown in Scheme 2. Interestingly, an attempt to ambush the carbocation from alcohol 32 with NaBH4 [4] was unsuccessful and gave only 9-phenylfluorene [32].
Despite the intervening years, this simple TFA-promoted Friedel-Crafts alkylation of benzylic alcohols has not been pursued. We now describe this alkylation as a simple alternative to the nuTrifluoroacetic merous other methods that have been reported for the benzylic alkylation of arenes with benzyl alcohols (e.g., HF [5], TsOH [6], Nafion-H [7] other cation-exchange resins [8], Sc(OTf)3 [9-11] NiOPO4·3H2 O [12,13], La(OTf)3 O [10], Yb(OTf)3 [10,11] TfOH [10], Hf(OTf)4 [10], rare earth (III) (OSO2C8F17)3 [14] zeolites [15-17], IrCl3·4H2O [18], Ir-Sn complex [19,20], 12-tungstophosphoric acid [21], I2 [22] IrCp* complexes [23], MoO6 [24], NaHSO4/SiO2 [25], Ph3P(OTf)2 [26], Fe(III)-porphyrin [27] BF3-H2O [28], Hf0.5[TEAPS] PW12O40 [29], choline chloride-(TfOH)2 O [30], and B(C6F2)3 [31]. Our method is eco-friendly as the only by-product is water and the TFA can be recycled. A summary of our results is depicted in Table 1. The reactions are not optimized. For example, in one case a 10-fold excess of TFA afforded a 93% yield of 17. In addition, we observed the benzylic alcohol and related alkylation shown in Scheme 2. Interestingly, an attempt to ambush the carbocation from alcohol 32 with NaBH4 [4] was unsuccessful and gave only 9-phenylfluorene [32]. Note: A: Reactions were conducted with a 5-fold excess of arene and TFA as solvent at reflux; B: Yield of distilled product based on benzyl alcohol 10 or 25; C: A ten-fold excess of TFA and 2 grams of benzyl alcohol (10); D: Equimolar amounts of naphthalene (22) and benzyl alcohol (22); E: Yield of crude product.
Note: A: Reactions were conducted with a 5-fold excess of arene and TFA as solvent at reflux; B: Yield of distilled product based on benzyl alcohol 10 or 25; C: A ten-fold excess of TFA and 2 grams of benzyl alcohol (10); D: Equimolar amounts of naphthalene (22) and benzyl alcohol (22); E: Yield of crude product.


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.