Advance of the Chemical Components and Biological Activities of Ajuga decumbens Thunb
Introduction
The genus Ajuga, belongs to the family of Labiatae, commonly known as “Bugleweed or Ground pine” [1] which distributed mainly in the temperate regions Europe, Asia and Africa [2] where most its species can be found, but only two of Ajuga species are available in southeastern Australia [3-4]. Pharmacological studies revealed that Ajuga possessed, have been used as anti-inflammatory agents, anti-cancer, astringent, anthelmintic, hypoglycemic, febrifuge diuretic, insecticidal properties and antifungal [5-11]. Ajuga decumbens is used as a folk medicine for the treatment of all kinds of ailment in ancient, most especially in China and Japan [12-15]. This review shows previous research on bioactive compounds of A. decumbens and biological activities of both the extracts and isolated compounds from this medicinal plant.
A. decumbens commonly found in stream sides, wet grassy slopes in Anhui, Fujian, Guangdong, Hainan, Hubei, Hunan, Jiangsu, Jiangxi, Qinghai, Sichuan, Taiwan, Yunnan, Zhejiang of China and some parts in Japan and Korea [16].
A. decumbens is an annual herbaceous flowering plant, which grow to 10-30 cm height with white villous on the young parts of the stem, numerous basal leaves longer than stem leaves, floral leaves are bractlike and lanceolate, with funnel- shape calyx and corolla tubular, straight, basally slightly swollen. Flowering starts from March and lasts to the end of July. The fruiting season starts from May and continues till November [16].
There are bioactive compounds isolated from A. decumbens, including diterpenoids, iridoid glycosides, triterpenoids, sesquiterpenoids, phytoecdysteroids, withanolides and other essential compounds, which are responsible for its effective biological activities.
Ajugacumbin H (17) was identified from the chloroform extracts of A. decumbens [24]. 15-epi-lupulin A (18), 6-O-deacetylajugamarin (19), Ajugadecumbenins A (20) and Ajugadecumbenins B (21) were discovered [25].
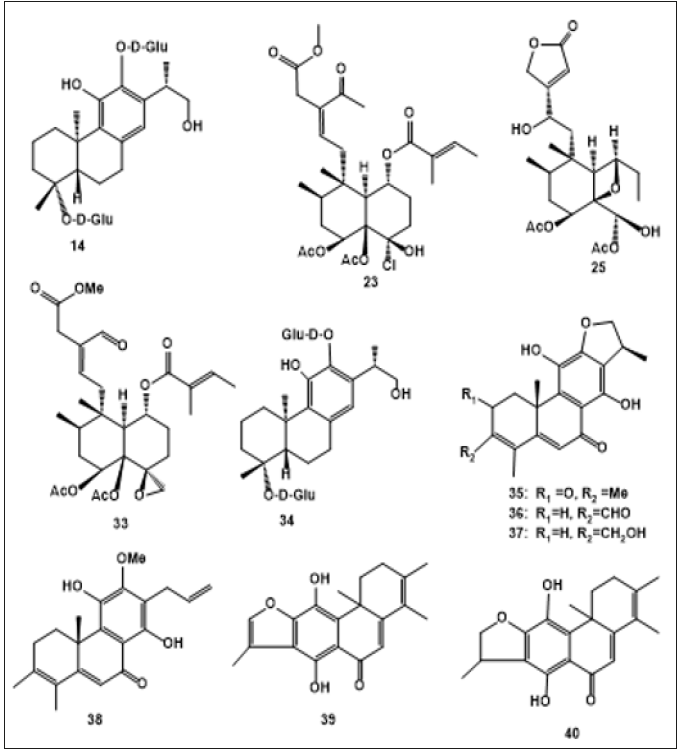
Ajugamarin A1 chlorhydrin (22) was isolated from the whole plant of A. decumbens [26]. Sun et al. also isolated ten neoclerodane diterpenoids, including 6α,19- diacetoxy-4α- hydroxy-1β-tigloyloxyneo-clerod12-en-15-oic acid methyl ester-16-aldehyde (23), (12s)-18,19-diacetoxy-4α,6α,12- trihydroxy-1β t igloyloxy-neo-clerod-13-en-15,16-ol ide (24), (12S)-1α,19-epoxy-6α,18-diacetoxy-4α,12-dihydroxyneoclerod- 13-en-15,16-olide (25), (12s)-6α,19-diacetoxy-18 chloro-4α-hydroxy-12-tigloyloxy-neo-clerod-13-en-15,16-olide (26), (12s,2′′s)-6α,19-diacetoxy18-chloro-4α-hydroxy-12-(2- methylbutanoyloxy)-neo-clerod-13-en-15,16-olide (27), 4α,6α- dihydroxy-18-(4′-methoxy-4′-oxobutyryloxy)-19-tigloyloxy-neoclerod- 13-en-15,16-olide (28), Ajugaciliatin J (29), Ajuganipponin B (30), Ajugamarin A1(31) , Ajugarin I (32) [27-28]. Lv et al. reported Ajugacumbin J (33) as a new neo-clerodane diterpene [29]. Diglucopyranoside (34) and Ajugaside (14) were isolated from the whole plants of A. decumbens as a newly detected abietane [30]. Wang et al. isolated Ajudecumin A (35), Ajudecumin B (36), Ajudecumin C (37) and Ajudecumin D (38) as newly rearranged abietane hydroquinones, with Ajuforrestin A (39) and Ajuforrestin B (40) as known abietanes from the aerial parts of A. decumbens [18] (Figure 1).
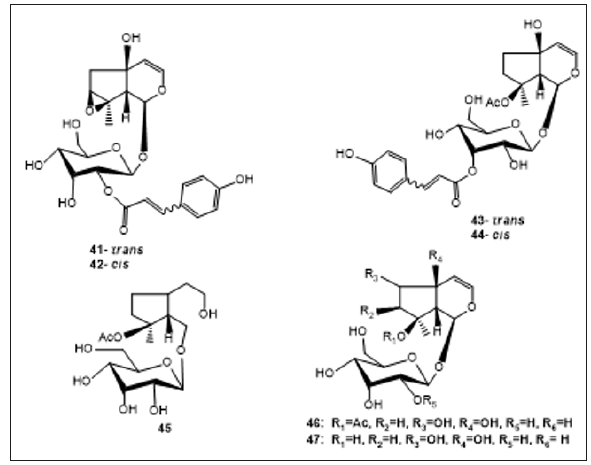
 Iridoid Glycosides: Iridoid glycosides are other predominant compounds of A. decumbens, Iridoid glycosides in plant contribute to defense against external factors such as herbivores or infection by microorganisms. Takeda et al., isolated Decumbeside A (41), Decumbeside B (42), Decumbeside C (43), Decumbeside D (44), reptoside (45) and 8-O -Acetylharpagide (46) from Methanol extract of A. decumbens [31]. Three Iridoid glycosides were reported to be presence in A. decumbens out of seven compounds isolated and elucidated [30]. Wen et al., reported the contents of 8-O-acetylharpagide (46) and harpies (47) in A. decumbens by HPLC-ELSD [32] (Figure 2).
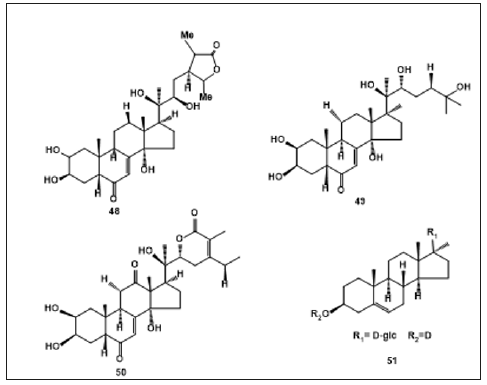
Iridoid Glycosides: Iridoid glycosides are other predominant compounds of A. decumbens, Iridoid glycosides in plant contribute to defense against external factors such as herbivores or infection by microorganisms. Takeda et al., isolated Decumbeside A (41), Decumbeside B (42), Decumbeside C (43), Decumbeside D (44), reptoside (45) and 8-O -Acetylharpagide (46) from Methanol extract of A. decumbens [31]. Three Iridoid glycosides were reported to be presence in A. decumbens out of seven compounds isolated and elucidated [30]. Wen et al., reported the contents of 8-O-acetylharpagide (46) and harpies (47) in A. decumbens by HPLC-ELSD [32] (Figure 2).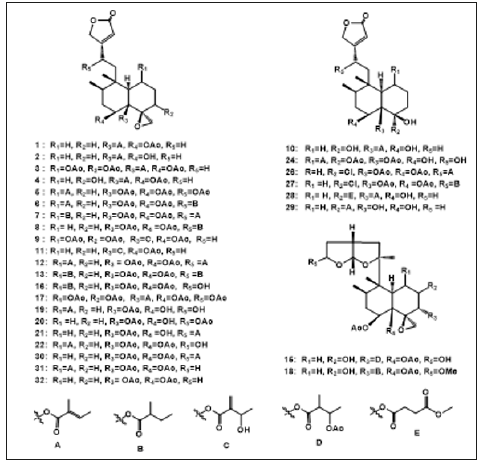 Phytoecdysteroids: Phytoecdysteroids are plant-derived cystoid’s. Phytoecdysteroids are class of triterpenoids that plants synthesize for protection against phytophagous (plant eating) insects. Phytoecdysteroids were also presence in A. decumbens. Cymserine (48) and Ecdysterone (49) are usually the most abundant phytoecdysones in the genus Ajuga, were isolated from the whole part of this medicinal plant [33]. Koreeda et al. reported the presence Ajugalactone (50) from A. decumbens [34]. Eight phytoecdysteroids were also discovered from the plant [17]. Guo et al. Isolated β-sitosterol 3-O-β-D-glucopyranoside (51) from whole plant of A. decumbens [35] (Figure 3).
Phytoecdysteroids: Phytoecdysteroids are plant-derived cystoid’s. Phytoecdysteroids are class of triterpenoids that plants synthesize for protection against phytophagous (plant eating) insects. Phytoecdysteroids were also presence in A. decumbens. Cymserine (48) and Ecdysterone (49) are usually the most abundant phytoecdysones in the genus Ajuga, were isolated from the whole part of this medicinal plant [33]. Koreeda et al. reported the presence Ajugalactone (50) from A. decumbens [34]. Eight phytoecdysteroids were also discovered from the plant [17]. Guo et al. Isolated β-sitosterol 3-O-β-D-glucopyranoside (51) from whole plant of A. decumbens [35] (Figure 3).Figure 3:
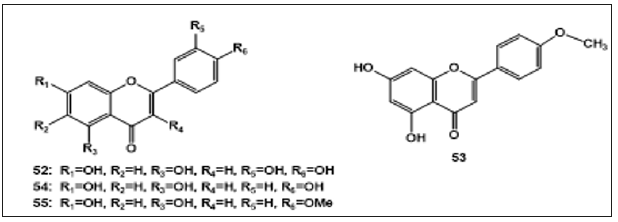
 Flavonoids: Studies have shown that various flavonoids are embedded in A. decumbens. Luteolin (52) was isolated from the ethanol extract of A. decumbens [36]. Guo et al., identified Dihydroxy- 4-methyl flavone (53) from its methanol extract [35]. Apigenin (54) was reported be presence in A. decumbens [26]. Acacetin (55) was obtained from the aerial parts of this medicinal plant [18] (Figure 4).
Flavonoids: Studies have shown that various flavonoids are embedded in A. decumbens. Luteolin (52) was isolated from the ethanol extract of A. decumbens [36]. Guo et al., identified Dihydroxy- 4-methyl flavone (53) from its methanol extract [35]. Apigenin (54) was reported be presence in A. decumbens [26]. Acacetin (55) was obtained from the aerial parts of this medicinal plant [18] (Figure 4).
 Flavonoids: Studies have shown that various flavonoids are embedded in A. decumbens. Luteolin (52) was isolated from the ethanol extract of A. decumbens [36]. Guo et al., identified Dihydroxy- 4-methyl flavone (53) from its methanol extract [35]. Apigenin (54) was reported be presence in A. decumbens [26]. Acacetin (55) was obtained from the aerial parts of this medicinal plant [18] (Figure 4).
Flavonoids: Studies have shown that various flavonoids are embedded in A. decumbens. Luteolin (52) was isolated from the ethanol extract of A. decumbens [36]. Guo et al., identified Dihydroxy- 4-methyl flavone (53) from its methanol extract [35]. Apigenin (54) was reported be presence in A. decumbens [26]. Acacetin (55) was obtained from the aerial parts of this medicinal plant [18] (Figure 4).Figure 4:
 Sesquiterpenoids: Few of Sesquiterpenoids are reported, Glecholone (56), (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3- one(57), (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9- one (58), (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one (59) and 6-Hydroxy-4,7-megastigmadiene-3,9-dione (60) were isolated from the aerial parts of A. decumbens [18]. Sun et al., Isolated Loliolide (61) from the whole plant of A. decumbens [26] (Figure 5).
Sesquiterpenoids: Few of Sesquiterpenoids are reported, Glecholone (56), (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3- one(57), (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9- one (58), (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one (59) and 6-Hydroxy-4,7-megastigmadiene-3,9-dione (60) were isolated from the aerial parts of A. decumbens [18]. Sun et al., Isolated Loliolide (61) from the whole plant of A. decumbens [26] (Figure 5).
 Sesquiterpenoids: Few of Sesquiterpenoids are reported, Glecholone (56), (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3- one(57), (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9- one (58), (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one (59) and 6-Hydroxy-4,7-megastigmadiene-3,9-dione (60) were isolated from the aerial parts of A. decumbens [18]. Sun et al., Isolated Loliolide (61) from the whole plant of A. decumbens [26] (Figure 5).
Sesquiterpenoids: Few of Sesquiterpenoids are reported, Glecholone (56), (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3- one(57), (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9- one (58), (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one (59) and 6-Hydroxy-4,7-megastigmadiene-3,9-dione (60) were isolated from the aerial parts of A. decumbens [18]. Sun et al., Isolated Loliolide (61) from the whole plant of A. decumbens [26] (Figure 5).Biological Activities
Anti-Cancer Activity: Takasaki et al. conducted in vitro inhibitory effects of the isolated compounds (Ajugaside A (14), Reptoside (45), 8-O-acetylharpagide (46), Harpagide (47), Galactosylmartynoside (62), Martynoside (63) and Darendoside B (64) on Activation of the Epstein–Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate induced phosphorylation were evaluated, the results revealed that 8-O-Acetylharpagide (46) displayed the strongest inhibitory effect on EBV activation induced by TPA [27-30]. 8-O-acetylharpagide (46) also displayed inhibitory effect on hepatic carcinogenesis using N-nitrosodiethylamine (DEN) as an initiator and phenol-barbital (PB) as a promoter [31-37]. Extracts of A. decumbens displayed anticancer and antimetastatic effects towards breast cancer through regulating the expression of MMPs and TIMPs [38]. Ajudecumin A (35), Ajudecumin B (36) and Ajudecumin C (37) showed inhibitory effect on the proliferation of human breast cancer MCF-7 cells [18]. High and moderate dosage of total flavonoid of A. decumbens significantly lower the expression of serum Interleukin-1(IL-1) and Tumor necrosis factor alpha (TNF-α), When compared with control [39]. Water extracts of A. decumbens significantly inhibited the increase of HepG2 cells in a dose-dependent manner [40]. Wang et al., reported that A. decumbens combined with Poria cocos have the ability to inhibit breast cancer cell invasion by targeting the expression of Mitogen-activated protein kinase (MAPK) and Epithelial-Mesenchymal Transition [41]. Iridoid in A. decumbens was investigated on the metastasis of invasive breast cancer cells and its related mechanism. The result showed that A. decumbens inhibited breast cancer cell invasion by suppressing the MAPK/ERK pathway as well as reversing the EMT [42,43] (Figure 6).
Anti-Cancer Activity: Takasaki et al. conducted in vitro inhibitory effects of the isolated compounds (Ajugaside A (14), Reptoside (45), 8-O-acetylharpagide (46), Harpagide (47), Galactosylmartynoside (62), Martynoside (63) and Darendoside B (64) on Activation of the Epstein–Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate induced phosphorylation were evaluated, the results revealed that 8-O-Acetylharpagide (46) displayed the strongest inhibitory effect on EBV activation induced by TPA [27-30]. 8-O-acetylharpagide (46) also displayed inhibitory effect on hepatic carcinogenesis using N-nitrosodiethylamine (DEN) as an initiator and phenol-barbital (PB) as a promoter [31-37]. Extracts of A. decumbens displayed anticancer and antimetastatic effects towards breast cancer through regulating the expression of MMPs and TIMPs [38]. Ajudecumin A (35), Ajudecumin B (36) and Ajudecumin C (37) showed inhibitory effect on the proliferation of human breast cancer MCF-7 cells [18]. High and moderate dosage of total flavonoid of A. decumbens significantly lower the expression of serum Interleukin-1(IL-1) and Tumor necrosis factor alpha (TNF-α), When compared with control [39]. Water extracts of A. decumbens significantly inhibited the increase of HepG2 cells in a dose-dependent manner [40]. Wang et al., reported that A. decumbens combined with Poria cocos have the ability to inhibit breast cancer cell invasion by targeting the expression of Mitogen-activated protein kinase (MAPK) and Epithelial-Mesenchymal Transition [41]. Iridoid in A. decumbens was investigated on the metastasis of invasive breast cancer cells and its related mechanism. The result showed that A. decumbens inhibited breast cancer cell invasion by suppressing the MAPK/ERK pathway as well as reversing the EMT [42,43] (Figure 6).
Antifeedant Activity: Ajugapitin (15) which is also known as Clerodendrin D was discovered from the leaves of A. decumbens as a feeding stimulant for Athalia rosae ruficornis [23]. Min et al. isolated four Diterpenoids (Ajugacumbins A, B, C and D) from the ethanol extract of A. decumbens, these isolated compounds displayed inhibition against the growth of insects [19]. Ajugacumbins E (9) and Ajugacumbins F (10) from A. decumbens showed insecticidal activities [21]. Ajugacumbins G (11) from this plant also exhibited growth-inhibitory activities against insects [22] (Figure 7).
Figure 7:
 Anti-Inflammatory Activity: Ajugacumbin J (33) and Ajugacumbin D (4) isolated from the whole plant medicinal plant exhibited inhibitory activities of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC50 s of 46.2 and 35.9mM, respectively [29]. Ono et al. examined the effects of ethanolic extracts of A. decumbens on nitric oxide (NO) production, expression of inducible nitric oxide synthase (iNOS), osteoblast and osteoclast activities. The results showed that ethanolic extracts inhibited expression of iNOS which caused concentration dependent inhibition of NO production, prevented brittle bones in ovariectomized mice and swelling of the left hind ankle in adjuvant induced arthritic rats [14] (Table 1).
Anti-Inflammatory Activity: Ajugacumbin J (33) and Ajugacumbin D (4) isolated from the whole plant medicinal plant exhibited inhibitory activities of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC50 s of 46.2 and 35.9mM, respectively [29]. Ono et al. examined the effects of ethanolic extracts of A. decumbens on nitric oxide (NO) production, expression of inducible nitric oxide synthase (iNOS), osteoblast and osteoclast activities. The results showed that ethanolic extracts inhibited expression of iNOS which caused concentration dependent inhibition of NO production, prevented brittle bones in ovariectomized mice and swelling of the left hind ankle in adjuvant induced arthritic rats [14] (Table 1).
Figure 7:
 Anti-Inflammatory Activity: Ajugacumbin J (33) and Ajugacumbin D (4) isolated from the whole plant medicinal plant exhibited inhibitory activities of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC50 s of 46.2 and 35.9mM, respectively [29]. Ono et al. examined the effects of ethanolic extracts of A. decumbens on nitric oxide (NO) production, expression of inducible nitric oxide synthase (iNOS), osteoblast and osteoclast activities. The results showed that ethanolic extracts inhibited expression of iNOS which caused concentration dependent inhibition of NO production, prevented brittle bones in ovariectomized mice and swelling of the left hind ankle in adjuvant induced arthritic rats [14] (Table 1).
Anti-Inflammatory Activity: Ajugacumbin J (33) and Ajugacumbin D (4) isolated from the whole plant medicinal plant exhibited inhibitory activities of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC50 s of 46.2 and 35.9mM, respectively [29]. Ono et al. examined the effects of ethanolic extracts of A. decumbens on nitric oxide (NO) production, expression of inducible nitric oxide synthase (iNOS), osteoblast and osteoclast activities. The results showed that ethanolic extracts inhibited expression of iNOS which caused concentration dependent inhibition of NO production, prevented brittle bones in ovariectomized mice and swelling of the left hind ankle in adjuvant induced arthritic rats [14] (Table 1). Anti-hyperlipidemic Effect: Xiaoming et al. reported that both the water and alcohol extracts of A. decumbens significantly showed reduction in the lipid levels in the blood, by administering the extracts on hyerlipemia mouse model [44].
Anti-hyperlipidemic Effect: Xiaoming et al. reported that both the water and alcohol extracts of A. decumbens significantly showed reduction in the lipid levels in the blood, by administering the extracts on hyerlipemia mouse model [44].A. decumbens is extensively used as traditional medicine for curing different ailments. Previous studies showed that both the isolated compounds and extracts possessed a wide spectrum of biological activities, such as anti-cancer, antioxidant, antifeedant, antibacterial, anti-inflammatory, antihyperlipidemic, anticholinesterase and cytotoxicity activities. All these pharmaco dynamic basis supported the use of A. decumbens as traditional medicines. However, although some structures of compounds were isolated and the relevant biological activities were tested, more compounds can be discovered and the medicinal values also need to be examined, such as analgesic, anthelmintic, cardioprotective and hypoglycaemic effects. In vivo experiments and toxicity of isolated bioactive compound and extracts should be overemphasized in order to be saved from consumptions. Researchers should focus on identification and quantification the bioactive compounds responsible for each biological activity that A. decumbens displays. There should adequate studies on elucidation of the molecular mechanisms underlying the activity of these bioactive compounds, which can lead to use of this plant extracts for drug development.
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.