In vitro Assessment of Anti-aging Properties of Syzygium cumini (l.) Leaves Extract
Syzygium cumini (L.) Skeels (Myrtaceae) is a plant originating from the tropics, in particular, from India, Thailand, Philippines and Madagascar, and it is also found in several states of the southeast, northeast and north regions of Brazil [1,2]. S. cumini known for its hepatoprotective, anti-allergic, hypoglycaemic, hypolipidaemic, anti-fungal, antiviral, anti-inflammatory and antioxidant properties, which are attributed to the presence of bioactive compounds in different parts of the plant [3-9]. The chemical composition of S. cumini leaves revealed the presence of phenolic compounds such as gallic acid, betulinic acid, myricetin, ellagic acid, chlorogenic acid, and quercetin, besides other components such as canferol, methyl gallate and nilocitin [10-16]. Phenolic compounds have received much attention lately, as they are natural inhibitors of oxidation and a source of broad-spectrum natural sunscreens [17-22]. It has been reported that there are plenty of opportunities in the market for these natural ingredients because they have a positive reputation to be readily accepted [23]. Furthermore, pieces of evidence show that topically applied antioxidants can be used for protection against sun damage [24,25], healing of wounds [23], as well as can act as antiinflammatory agents [26,27]. To achieve a safe treatment strategy using natural products, brine shrimp lethality assay can be used in toxicology tests, which screens a large number of extracts for drug discovery in medicinal plants [28-31], and marine products [32]. In vitro toxicology analysis using human keratinocyte cells (HaCaT cell line) has also been used as an important alternative for early toxicity assessment. Moreover, keratinocytes are the most common cell type in the epidermis (about 90%), and thus they are used frequently as an epidermal model system [33,34]. Keeping the above data in mind, the purpose of the present study was to investigate the anti-aging potential and toxicity of ethanol extract from leaves of S. cumini well as its total phenolic and flavonoid contents.
Plant Material
The leaves of S. cumini were collected in April 2016 from the Federal University of Juiz de Fora (UFJF) - Juiz de Fora, Minas Gerais, Brazil, and identified by Research Fátima Regina Gonçalves Salimena, UFJF, Brazil – voucher specimen (CESJ 35342) from Leopoldo Krieger Herbarium of UFJF. The collected leaves were dried at 40°C in a kiln and then reduced to powder using a knife mill (TA-2; Metvisa, Brazil).
Preparation of the Extract
Ethanol extract was obtained from 10g of dried leaves were weighed and macerated in ethanol for 72h at room temperature. The residue was removed by filtration, and the extract was evaporated to dryness at a lower temperature (<40°C) under reduced pressure in a rotary evaporator (R-114; Buchi, Switzerland), obtained yield of 9.4% w/w. The material was stored protected from light in airtight containers with the cap at −20°C to use.
Phytochemical Assay
Secondary metabolites (flavonoids, tannins, coumarins, heterosides, saponins, alkaloids and anthraquinones) were investigated in the leaves of S. cumini through reactions of functional groups of the molecule according to Matos [35].
Total Phenolic Content
The total phenolic content was determined by the spectrophotometric method, as described previously [36], with few modifications, using Folin–Ciocalteu reagent. Briefly, we added 50µl of extract dissolved in ethanol solution (10 mg/ml), 250 µl of Folin–Ciocalteu reagent, 500µl of 20% sodium carbonate and 4.2 ml of distilled water. This reaction proceeded at room temperature, in the dark, for 30 min. Then, the absorbance was read on a spectrophotometer (Multiskan GO, Thermo Scientific, USA) at λ = 765 nm. Gallic acid (Sigma-Aldrich, USA) was used a standard, and its ethanolic solutions (25–700 μg/ml) were used to construct a standard curve for determining the extract’s phenolic content. Tests were performed in triplicate, and the results were expressed in milligram of gallic acid equivalents per 100 mg dry extract. The total phenolic content of the extract was determined by extrapolation of the analytical curve constructed from the gallic acid standard.
Total Flavonoid Content
Determination of flavonoids was performed according to Quettier-Deleu [37], with modifications, through reaction with aluminum chloride, resulting in a yellow complex, whose absorbance can be measured in a spectrophotometer. The medium contents of flavonoids were rutin (Sigma-Aldrich, USA) equivalents, which were used as a standard for the construction of the calibration curve (2–30μg/ml). To quantify the flavonoid content, 2.5ml of extract was semi-purified in ethanol solution (10mg/ml) by 4 adding aliquots of 1 ml of chloroform and 1.5ml of distilled water. The resulting solutions were mixed and centrifuged for 3 min at 2,465 x g at room temperature. The aqueous phase (20µl) was mixed with distilled water (99µl), 8% aluminum chloride (2µl), pyridine methanol solution (100µl) and glacial acetic acid (6µl). Following incubation for 15 min (room temperature), the absorbance was measured in a spectrophotometer (λ = 405 nm). Tests were carried out in triplicate, and the results were expressed as milligram rutin equivalents per 100mg dry extract.
Antioxidant Activity
The scavenging activity of S. cumini leaves was measured according to the 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) method, as described previously by Sreejayan and Rao [38], with minor modifications. Briefly, the sample (50µl) at different extract concentrations (0.97–250 μg/ml) was added to each well of a 96- well microplate and mixed with 150µl of 50µM DPPH in ethanol solution. The reaction mixture was then kept for 30 min in the dark at room temperature. Then, the absorbance was measured in a spectrophotometer at λ = 510nm against the negative control (ethanol). Ascorbic acid and Resveratrol (Sigma-Aldrich, USA) was used as positive controls at the same concentrations. Inhibition of DPPH radical was calculated using the following equation: IC50 (%) = 100 × (A0 – As)/A0, where A0 and As are the values for the absorbance of the negative control and the absorbance of the sample, respectively. The IC50 value was calculated from the straight-line equation of the linear dispersion graph and represents the extract concentration that inhibits 50% of DPPH radical. All tests were performed in triplicate
Photoprotection Assay
The photoprotection capacity of S. cumini leaves was measured according to Polonini and colleagues [39], conducted in UV-2000S Ultraviolet Transmittance Analyzer (Labsphere, USA) equipped with two photodiode array spectrographs and integrating sphere. Xenon flash lamp, that emits a continuous spectrum of radiation with no peaks, supplies energy in the spectral range (290–450 nm) with an increment step of 1 nm and has a low irradiance, such that photostability of the product is not unduly challenged. Moreover, analyzes were performed on square-shaped (50 × 50 mm) polymethylmethacrylate (PMMA) Helioplate™ HD6 (HelioScreen, France) plates with roughened surface one side (Sa ≈ 6 μm) were used as the substrate for the determination of sunscreen protection factor (SPF) by diffuse transmittance spectrophotometry. Electronic analytical balance (AY - 220, Shimadzu, Japan) was used in the compounding of the sunscreen products. The samples were accurately weighed on the PMMA plate surface to satisfy the application rate of 1.3mg/cm2 in each plate.
Then, the products were spread over the whole surface using a fingertip covered with a vinyl glove and pre-saturated with the product. For each product, three plates were prepared, which were kept protected from light exposure in a dark chamber at room temperature (≈ 20 °C) for 15 min. Following, the transmission of UV radiation through the sample was measured from 290 to 450 nm at 1nm intervals on nine different sites of each plate. The blank was prepared using HD6 plates covered with 15μl of glycerine because of its non-fluorescence and UV transparency. Then, UVB protection efficacy as SPF; and UVA protection efficacy a UVA/UVB Ratio and Critical Wavelength were calculated, as equations described previously [40]. The validity of experiments were obtained using the Cosmetics Europe Reference Sunscreen S2 (determined SPF=18±1.5, UVAPF = 12 ± 1.1, λcritical = 381 nm, and UVA/UVB ratio = 0.88). During UVAPF analysis, two UV spectrums were obtained, after exposing the plates to calculated UV dose. Before, UVAPF, UVA/UVB ratio and SPF label as the critical wavelength were calculated. Data are expressed as mean of 27 measures for the lotion containing 15% of S. cumini extract
Tyrosinase Inhibition
Tyrosinase inhibition qualitative enzymatic reaction screening was performed according to the protocol described previously [41], with some modifications. S. cumini extract was diluted in the microplate wells to concentrations of 100, 80, 40, 20 and 10µg/ ml with 2.5% DMSO, and the kojic acid (positive control, SigmaAldrich, USA) was diluted to concentrations of 10, 5, 2.5, 1.25 and 0.625µg/ml. Then, aliquots of 10µl of a solution composed of 125 U/ml of mushroom tyrosinase (Sigma-Aldrich, USA) were added in 96-well microplates, 70µl of phosphate buffer solution (pH 6.8) and 60µl of S. cumini extract was also added. For the negative control, 60µl of 2.5% DMSO was added. The absorbances of the microplate wells were read on a spectrophotometer at λ = 510 nm (T0). Then, the microplates were incubated at 30 ± 1 °C for 60 min, and the absorbances were measured again (T1). An additional incubation for 60 min at 30 ± 1 °C was carried out, after which a new spectrophotometric reading was taken (T2). The inhibitory percentage at the two time points (T1 and T2) was obtained according to the equation: IA(%) = 100 x (C-S)/C, where IA (%) is inhibitory activity; C = negative control absorbance; S =sample or positive control absorbance (absorbance at time T1 or T2 minus the absorbance at time T0). The analytical curve was plotted between tyrosinase inhibition activity percentages at each time point and the concentrations of the extract/positive control. Using the equation of the line, the inhibitory activity at 50% (IA50, in µg/ ml) was calculated.
Cell Viability Assay
Human keratinocyte cells (HaCaT cell line) were cultured as adherent monolayers in Dubelcco’s Modified Eagle`s Medium (DMEM) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100µg/ml streptomycin and 10 mM HEPES and maintained at 37 °C in a 5 % CO2 humidified atmosphere at pH=7.4. The cell viability study was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay [42]. Briefly, HaCaT cells was seeded in 96-well plates at a density of 5 × 104 cells/ml in 100µl of medium per well. After 24 h of incubation, the culture medium was replaced by fresh medium with the treatments. Sextuplicate wells were treated with S. cumini extract at concentrations ranging from 500 – 7.81µg/ml. The plates were incubated at 37 °C in 5 % CO2. A control experiment was performed under the same conditions, but without cell treatment. After 24 h, the medium was removed and 200µl of DMEM with 50µl of MTT (5 mg/ml) dye solution was added, followed incubation for 3 h at 37 °C. The precipitated formazan was then dissolved in DMSO, and the absorbance was measured at λ = 570 nm using a microplate reader. Cell viability (%) was expressed as a percentage of the absorbance of non-treated control cells. IC50 value is the concentration of sample required to inhibit 50% of the cell proliferation and was calculated plotting the percentage survival vs. the concentrations, using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA).
Brine Shrimp Lethality Assay
The brine shrimp lethality assay was performed according to the protocol described [43], with some modifications. Brine shrimp cysts were obtained from Maramar Aquacultura (Cabo Frio, Rio de Janeiro, Brazil) and incubated in artificial seawater and exposed to a 60-W lamp at pH 8–9. After 48 h of incubation at room temperature (22 –29 °C), the active nauplii free from eggshells (10 units) was collected and added to each set of wells containing crude ethanol extract dissolved in 2.5% DMSO and made up to 5 ml total volume using artificial salt water. The extracts were tested in triplicate at 1000 – 10μg/ml. In addition, thymol and 2.5% DMSO were used as positive and negative controls, respectively (and artificial seawater a negative control). After 24 h, the number of survivors was counted using a binocular microscope, and the percentage of deaths was calculated. The lethal concentration 50 % (LC50 value) and the standard error were calculated by Probit analysis [44].
Statistical Analysis
The results were calculated as a mean ± standard deviation (SD). All experiments were analyzed by Analysis of Variance (ANOVA), and Tukey’s test was used as post hoc multiple comparisons between treatment group and control. P < 0.05, 0.01 and 0.001 were considered significant. The statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA).
Phytochemical screening of leaves of S. cumini showed the presence of flavonoids, tannins, triterpene heterosides, and saponins, which were similarly reported by Ayyanar [45]. Tannins and flavonoids are secondary metabolites classified as polyphenols, which are compounds that have broad-spectrum biological activities, including the ability to sequester free radicals [46]. Recently, there has been increasing interest in the natural antioxidants contained in the medicinal plants, which are candidates to prevent oxidative damage, including skin disorders [47,48]. The antioxidant and free-radical-scavenging property of flavonoids can be attributed to the high mobility of the electrons in the benzenoid nucleus and are well known for enhancing the wound healing process [49]. These properties can be easily applied to skin-related ailments as reducing the production of free radicals, prevent damaging the structure and functions of skin cells. Next, we investigated whether the treatment with ethanol extract of S. cumini could inhibit DPPH radical. Remarkably, in vitro treatment with S. cumini extract significantly induced scavenging activity (IC50 = 9.85 ± 0.51 μg/ml), when compared with to ascorbic acid (IC50 = 3.02 ± 0.09μg/ml) and resveratrol (8.05 ± 0.51 μg/ml) – used as positive controls. In order to evaluate the possible mechanisms involved in the antioxidant action of S. cumini, we next assessed the phenolic and flavonoid contents in the S. cumini extract.
The contents of total phenols and flavonoids of ethanol extract of S. cumini were 19.69 ± 0.87 and 1.21± 0.081 mg/100 mg, respectively. The results showed the good capacity of the S. cumini extract to act against the radical DPPH, which correlates with phenolic compounds contents, such as flavonoids. Therefore, our data confirm and largely extend previous data, which demonstrated that phenolic compounds, such as ferulic acid and catechins are responsible for the antioxidant activity of extracts from the leaves of S. cumini [50]. More recently, Gajera and colleagues demonstrated that the inhibition of DPPH radical scavenging activity was positively correlated with phenol constituents from fruit parts of indigenous S. cumini. Moreover, the same authors demonstrated that the seed and kernel of S. cumini extracts exhibited higher phenolics-gallic, catechin, ellagic, ferulic acids and quercetin, whereas the pulp evidenced higher with gallic acid and catechin, as α-amylase inhibitors [51]. Extending this idea, Chandran and colleagues reported that S. mundagam methanol extract showed therapeutic effects on type-2 diabetic complications in rats, which were also correlated with phenolic contents [52]. Next, we assessed whether the treatment with S. cumini could induce photoprotection capacity.
The results of Table 1 demonstrate that S. cumini extract exhibited a significant photoprotection capacity for a single UV-filter substance, as its SPF was equal to 13 ± 2.9. Moreover, the extract also showed a relevant UVA protection (UVA-PF = 5.0) and a critical wavelength value of 385nm (Table 1). It can be considered as a broad-spectrum filter, according to the Food and Drug Administration, and Colipa [53]. Finally, we determined the UVA/UVB ratio, which provides a good idea of which UV region is better blocked by the substances. The value (0.550) showed that S. cumini extract has an important UVA/UVB balance. To our knowledge, this is the first study reporting the photoprotection capacity of the extract of S. cumini leaves. Importantly, natural substances have been considered as potential sunscreen resources because of their absorption in the UV region and their antioxidant activity [54]. Green tea polyphenols; aromatic compounds isolated from lichens; glycosides of esculin and Murraya koenigii leaf are examples of natural substances evaluated for their sunscreen specifications [54].
Table 1: Photoprotection effects of ethanol extract from S. cumini leaves.
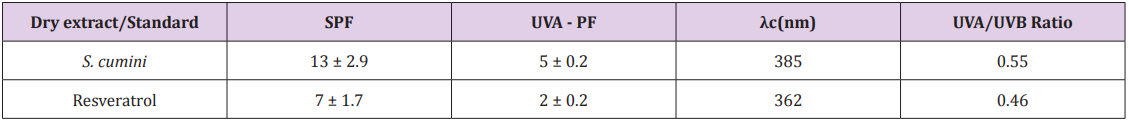
Note: SPF: sun protection factor; UVAPF: UVA protection factor; λc: critical wavelength. All results were expressed as mean of 27 determinations (3 plates vs. 9 individual readings) for lotion containing 15% of S. cumini extract, using Cosmetics Europe Reference Sunscreen S2 (SPF = 18 and UVA – PF = 12).
In addition, dried extract of Lippia sericea, Aloe barbadensis and Alpinia speciose have been described in their photoprotective properties. Relevantly, there is strong evidence that DNA-damaging ultraviolet (UV) light induces the accumulation of UV lightabsorbing flavonoids and other phenolic compounds in the dermal tissue of the plant body. This suggests the physiological function, although speculative, in light protection in plants and, of course, in humans [55]. There has been an increasing interest in the use of antioxidants in sunscreens to provide supplemental photoprotective activity. Taken together, we suggest that the photoprotective effects of S. cumini extract on sun damage are associated, in part, with antioxidant activity and phenolic compounds, especially flavonoid. For this reason, our result supports the use of antioxidants from S. cumini extract as new possibilities for the treatment and prevention of UV-mediated diseases. However, to date, we are not able to confirm whether S. cumini leaves show photoprotection effects in vivo. For this reason, future studies are needed to clarify this hypothesis. According to Alam et al. [56], hydroxyl groups of phenolic compounds could inhibit enzymatic activity through hydrogen bond formation with the active site of tyrosinase enzyme.
Some tyrosinase inhibitors act through binding of their hydroxyl groups to the active site of the enzyme, resulting in steric hindrance or conformation change. Moreover, the antioxidant activity may be one of the important mechanisms for tyrosinase inhibition activity. In this set of experiments, we investigated whether the treatment with leaf extracts of S. cumini could inhibit tyrosinase activity in vitro. As shown in Table 2, the ethanol extract from S. cumini leaves showed IA% lower than 50 % (Table 2). Furthermore, in the quantitative assay, it showed a tyrosinase inhibition capacity markedly lower than kojic acid – used as positive control (Table 2). Although this extract has shown relatively weak tyrosinase inhibition activity, it may act through a different inhibition pathway, which is not based on tyrosinase activity. Therefore, future studies will need to investigate whether S. cumini could inhibit this enzyme in epithelial tissue after sun damage, as well as confirm whether or not S. cumini directly modulates the tyrosinase activity and/or melanocytes during sun exposition. Human immortalized HaCaT keratinocyte cells closely resemble normal human keratinocytes and therefore represent a valuable in vitro cell model for testing the effects of natural products of cosmetic interest, as well as the cytotoxic effect of natural compounds [57].
Table 2: Tyrosinase inhibitory activity of ethanol extracts from S. cumini leaves.
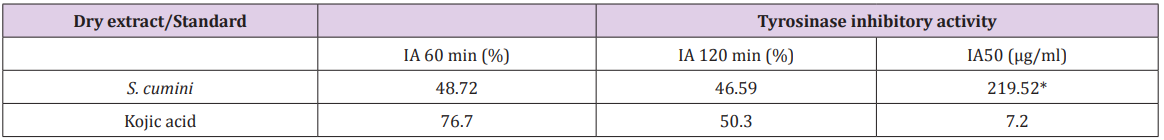
Note: The analytical curve was plotted between tyrosinase inhibition activity percentages at each time point and the concentrations of the extract/positive control. Using the equation of the line, the inhibitory activity at 50% (IA50, µg/ml) was calculated. *p<0.05 versus positive control group (one-way ANOVA followed by Tukey’s test). IA: inhibitory activity.
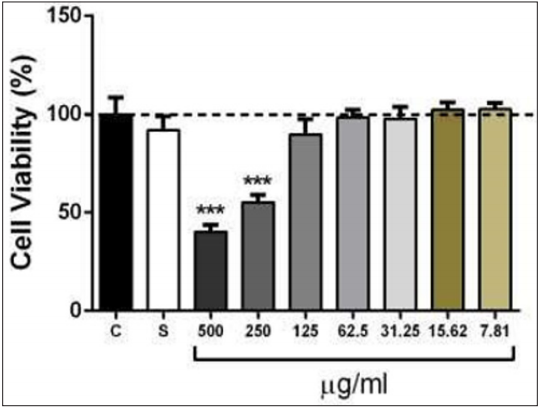
In this set of experiments, we investigated whether the treatment with ethanol extracts from S. cumini leaves could induce cytotoxicity effect in HaCaT keratinocyte cells, through MTT assay. Ethanol extract of S. cumini (125 – 7.81μg/ml) were not toxic, while the concentrations of 500 and 250μg/ml revealed modest cytotoxicity when compared to the control and solvent (DMSO) groups after 48 h incubation (Figure 1). Additionally, brine shrimp lethality assay has been widely documented as a screening method for the assessment of toxicity in mammals and humans [28,29]. Therefore, the acute toxic effect of the leaf extract of S. cumini was evaluated using an invertebrate in vivo model (A. salina). The LC50 exhibited by this extract was 616.50µg/ml (Table 3). According to the result, its toxicity to A. salina was far below the limit of 1,000µg/ml reported by Meyer et al. [43]. The mortality of brine shrimp after exposure to S. cumini extract was expected to be associated with bioactive compounds and not with starvation. This was confirmed by the absence of brine shrimp death in the negative control group. The brine shrimp toxicity results suggest that the extract does not have high toxicity compared to that of thymol standard (positive control) with LC50 of 42µg/ml. Taken together, as preliminary toxicological data suggest that S. cumini is quite safe and did not present any evidence of important cytotoxicity, although additional protocols are necessary to confirm this hypothesis. Moreover, the present data showing that S. cumini extract could prevent UV-induced skin damage, including inflammation and cellular toxicity in keratinocytes, support its utilization in cosmetic biopharmaceuticals.
Table 3: Preliminary toxicological effect of S. cumini extract against brine shrimp after 24 hours incubation

Note: The extracts were tested in triplicate at 1000 – 10μg/ml. Thymol and 2.5% DMSO were used as positive and negative controls, respectively. After 24 h, the number of survivors was counted using a binocular microscope, and the percentage of death was calculated. The lethal concentration 50 % (LC50 value) and the standard error were calculated by Probit analysis [44]. *p<0.05 versus positive control group (Student’s t-test).
Figure 1: Cytotoxicity effect of ethanol extracts from S. cumini leaves against human immortalized HaCaT keratinocyte cells. Sextuplicate wells were treated with S. cumini extract at concentrations ranging from 500 – 7.81µg/ml. The plates were incubated at 37 °C in 5 % CO2 . IC50 value is the concentration of sample required to inhibit 50% of the cell proliferation and was calculated by plotting the percentage survival vs. the concentrations. ***p < 0.001 versus the control group (one-way ANOVA followed by Tukey’s test).
In summary, data from the present study reveal that ethanol extract of S. cumini leaves showed marked phenolic compounds contents, especially flavonoid, and also displayed biological activities, deeming it an ideal cosmetic ingredient, including the DPPH radical scavenging (IC50 = 9.85 ± 0.51 μg/ml), tyrosinase inhibition (IA50 value of 219.52 μg/ml), as well as sun protection factor (13 ± 2.9) activities. Moreover, this extract showed low and moderate cytotoxicity in human keratinocytes and brine shrimp assay. Therefore, the use of this extract, alone or in combination with other active ingredients, may be of interest to the cosmetic industry, because of its anti-aging properties.
Antimicrobial Peptide Brevinin-2isb, New Drug Candidates Enhance the Innate Immune Response and Cured Caenorhabditis Elegans With Methicillin-Resistant Staphylococcus Aureus (MRSA)-https://biomedres01.blogspot.com/2021/01/antimicrobial-peptide-brevinin-2isb-new.html
More BJSTR Articles : https://biomedres01.blogspot.com


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.