Investigation of Different Materials as Acetone Sensors for Application in Type-1 Diabetes Diagnosis
Introduction
It is necessary to detect diseases at an early stage since this may minimize harmful effects on the human body. Diabetes, as the 7th leading cause of death in the United States, is a disease that is characterized by a heterogeneous group of blood disorders and can be indicated by high Blood Glucose (BG) levels [1,2]. Based on 2017 statistics, about 30.3 million people in the U.S. have diabetes, which is about 9.4% of the U.S. population [3]. Among those 30.3 million people, there were 23.1 million people diagnosed as diabetics. The remaining 7.2 million people were not diagnosed due to complicated reasons. One of major concerns is due to expensive and complicated diagnosing process [3]. So, it is necessary to provide an affordable, reliable, and convenient method which makes the segment of population to obtain diagnosis and treatment in time. There are many ways to screen diabetes in the early stage, such as the A1C test (a blood test that provides information about blood glucose levels) [4], the Fasting Plasma Glucose Test (FPG), and the Oral Glucose Tolerance Test (OGTT) [5]. These tests are accurate but inconvenient with relatively high cost.
Most recently, detection of Volatile Organic Compounds (VOCs) in exhaled breath has caused a lot attention since some of VOCs are found closely related to human health [6]. Therefore, some Volatile Organic Compounds (VOCs) have been identified as biomarkers for different diseases, such as carbon disulphide for Schizophrenia [7,8], isovaleric acid for urinary tract infections [9], dodecane, 4-methyl decane, and undecane for melanoma [10]. Besides the compounds listed above, breath acetone, has also received attention due to a good correlation between the concentration of acetone in exhaled breath and blood sugar level in human body [11,12]. This is because metabolic disorders can affect the concentration of acetone in human breath [13-17]. So, breath acetone can be used as a biomarker to diagnose diabetes. By measuring the concentration of acetone in human breath [17,18], it could provide a convenient, inexpensive, and non-invasive way for early-stage diabetes diagnosis and health condition monitoring. Generally, concentration of exhaled breath acetone is usually in the range of 0.3-0.9 Parts-Per-Million (ppm) for healthy humans and above 1.8 ppm for diabetic patients [18]. While, acetone concentration between 0.9 ppm to 1.8 ppm is considered as prediabetes. Thus, a breath analyzer to detect the concentration of breath acetone below 0.9 ppm could be an efficient way of screening diabetes at an early stage.
The nanostructured K2W7O22 (KWO) material [19], recently developed in our research group, shows a sensitive response to acetone at room temperature. The preliminary results indicate that such sensitive response is mainly due to the porous structure and excellent ferroelectric property [20] of KWO material. This could cause a strong surface interaction and effective charge transfer between sensing molecules and KWO. In this paper, we will briefly discuss the sensing performance of KWO to detect acetone. In the meanwhile, we choose other four materials: Pt-InN [13], Polypyrrole (PPy)-WO3 [21], Ni/InGaN/GaN [22], and Pd/TiO2 /Si [23], which all show good response to acetone. The main purpose of this paper is to study those five materials with different sensing conditions for their potential application in diabetes diagnosis and monitoring [24,25].
Experimental Section
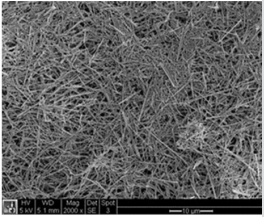
In this section, a brief description of KWO synthesis will be presented. In addition, KWO film preparation, equipment and procedure of a chemiresistive sensor based on KWO to detect acetone are discussed. The single crystalline nanostructured KWO was synthesized by a hydrothermal technique [26,27]. Briefly, a precursor solution containing Na2WO4 , oxalic acid, K2SO4 , and HCL is made. This solution is then put into a 30 mL autoclave for synthesis. KWO samples were grown at 225o C for 24 hours. The as-synthesized nanostructured KWO were dispersed in ethanol to form a suspension and drop-casted on glass substrates to form a thin film with about 10 µm in thickness. The morphology of the KWO film was studied with scanning electron microscopy (SEM) (Figure 1) and the film shows a highly porous structure made of a three-dimensional mesh of randomly orientated and interconnected nanorods. The average length of the nanorods is about several micron and the diameter of nanorod is about 10 nm, which are adjustable through synthesis conditions. Electric contact pads made of gold are sputtering deposition onto the thin film. The sensing response of KWO by exposing acetone is determined by measuring its resistance between the metal contacts with an electrometer (Keithley 6514). More information regarding KWO to detect acetone can be found from our published literatures [19,24,25].
Figure 1: Effects of soil salinity on leaf and root dry weights of two cotton cultivars at flowering and boll-forming stages, during 2013 and 2014. LS: low soil salinity; MS: medium soil salinity; HS: high soil salinity. Vertical bars represent ± standard error (n = 3). Bars labelled with different lowercase letters on open-square bars or uppercase letters on closed-square bars represent significant differences (P < 0.05).
Results
This section mainly discusses the sensing performance based on five materials towards acetone detection under different circumstances (temperature, concentrations, response time etc.)
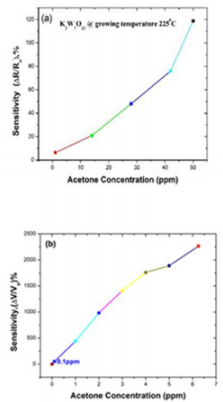
The material KWO (made in our group) is grown with thermal temperature at 225o C. Figure 2a shows the response of KWO to detect acetone with variable concentration from 0 ppm to 50 ppm at room temperature with RH ~ 30%. It was observed that the sensitivity is only 6.34% while acetone concentration is about 0.125 ppm. It is not optimal considering its potential clinic application. A much lower detection limit of acetone is needed. After we modified the signal collecting circuits, the KWO sensor can be used to detect quite low concentration of acetone, for example, 0.125 ppm with decent sensitivity. Figure 2b shows the response to acetone which concentration is from 0 to 6.25 ppm. The results indicated a detection limit can be up to 0.1 ppm with sensitivity around 50.75%. It is about 8 times higher than the sensitivity of 6.34% based on our previous sensing system. The results revealed that KWO is a promising sensing material to detect acetone.
Figure 2: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
PPy-WO3
The sensing response of PPy–WO3 (20%) hybrid nanocomposite sensor [21] to different concentration of acetone at operating temperature 90o C has been redrawn in Figure 3 (The graph is based on the data of Figure 2(b) in article [21]). As indicated in the Figure, the sensitivity of this sensor is quite low, which is less than 0.5% even when acetone concentration is 200 ppm.
Figure 3: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
InN and Pt-InN
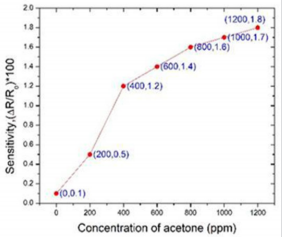
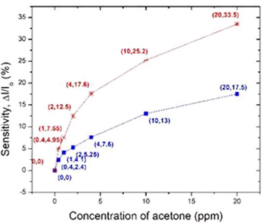
Kun Wei, had studied bare InN and Pt-InN sensors to observe the response of acetone gas at different operating temperatures (150o C, 175o C, and 200o C). A comparison of sensitivity of both the sensors from 0.4 ppm to 20 ppm at operating temperature 200o C is redrawn in Figure 4 (The data has taken from the graph of (2b and 4b) in article [13]). It was found that Pt-InN (red line) shows higher more sensitive to acetone compared to bare InN (blue line). The sensitivity of 4.95% was observed on Pt-InN while was almost two times higher than the sensitivity of bare InN, 2.4% while acetone concentration was 0.4 ppm.
Figure 4: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
Nanocrystalline TiO2
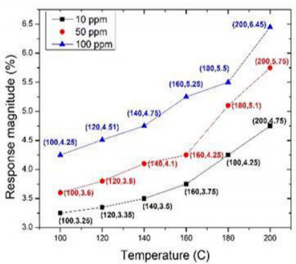
In article [23], nanocrystalline TiO2 based Metal Insulator Semiconductor (MIS) was used as the sensing material to detect different concentrations of acetone (10, 50, 100 ppm) from 100o C to 200o C. Figure 5 shows the response magnitude of TiO2 sensor for 10, 50, 100 ppm at three different temperatures (The data of Figure 4 has taken from the graph (6) in article [23]). The overall sensivities of TiO2 to acetone are no more than 5%. While, they observed that the sensing response can be improved a little bit while sensors were operating at higher temperature.
Figure 5: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
Ni/InGaN/GaN
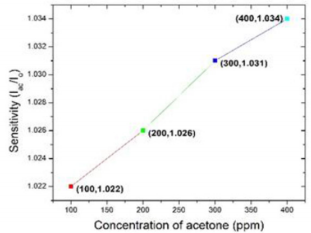
In article [22], Ni/InGaN/GaN heterostructure resistive sensor is used to detect acetone concentration from 100-400 ppm at fixed operating temperature 100o C. They measured the sensitivity in terms of ratio of current in presence of acetone vapor (Iac) to current in air (Iair). Figure 6 shows the sensitivity of the heterostructure sensor has a slight increase while the acetone concentration is higher, 1.034% on 400 ppm of acetone and 1.022% on 100 ppm of acetone (The data of Figure 6 has taken from the graph 4(a) of article [22]).
Figure 6: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
Discussion
Detection limit, sensitivity, and response time [28] are the most important parameters for evaluating the sensing performance of sensors. Detection limit is defined as the lowest quantity of substance that can be distinguished from the absence of that substance with a 99% confidence level [29]. Sensitivity is defined as the variation in current ratio for specific gas concentration. If Rgas and Rair are the resistance values of the sensor, then the sensitivity, S [13] is,
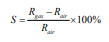
Response time [13] measures the time it takes for the relative current to change from 10% to 90% of the total current. In addition, sensitivity and response time can be represented in terms of voltage or current. Considering the purpose of a breath analyzer is to be used in a disease diagnosis fast response, quick recovery, high sensitivity, and low detection limits (a range of ppb to ppm) are all important factors. Since doping, operating temperature, configuration, and surface modification are usually used to optimize these parameters, sensing performance is relying on them as well. Below, the sensing performances based on five materials, PPy-WO3 , InN, Pd/TiO2 /p-Si, Ni/InGaN/GaN and KWO, with different doping, surface modification, and operating conditions have been discussed and summarized.
PPy-WO3 Hybrid Nanocomposites Sensor
As discussed earlier (Figure 3), Hoda et al. [21] investigated how a Polypyrrole (PPy)-WO3 hybrid nanocomposite with variable percentages of WO3 nanoparticles dispersed in a PPy matrix can be used as the sensing material to detect acetone. Their result showed that the PPy-WO3 (20%) hybrid nanocomposite has a fast response (<5s) to acetone vapor at an operating temperature of 90o C. Also, they observed the detection limit for PPy-WO3 (20%) was 0.37 ppm.
InN Gas Sensor
Kun Wei, investigated [13] how well an Indium Nitride (InN) gas sensor having a thickness of 10nm was able to detect acetone down to 0.4 ppm (Figure 4) at different operating temperatures: 150o C, 175o C, and 200o C. They used platinum as a catalyst, to increase current signals and reduce response times and found that Pt-InN has better response than Bare InN.
Pd/TiO2/p-Si MIS Sensor
Hazra, introduced a nanocrystalline TiO2 based sensor [23] that can detect acetone from 10 to 100 ppm (Figure 5). They prepared a TiO2 sensing layer-based MIS device structure that was operated between 100 to 200oC. They observed the response time was 7.7 sec at 100o C for 10 ppm. Also, the sensitivity is better while the sensor worked at higher temperature.
Ni/InGaN/GaN Heterostructure Sensor
Subhasis, developed a [22] Ni/InGaN/GaN based heterostructure resistive gas sensor (Figure 6). They exposed this sensor to 100 ppm acetone with an operating of bias 0.4 V at 373 K (100o C). In their analysis, they observed the response time of ~7.6-8.4 second and a corresponding recovery time of ~ 4.5-19.1 second.
KWO Sensor
The KWO sensor, as shown in Figure 2, can detect acetone concentration up to 0.1 ppm at room temperature (25o C) based on a chemiresisive sensing mechanism. The KWO nanorods exhibits excellent sensing performance to acetone [31].
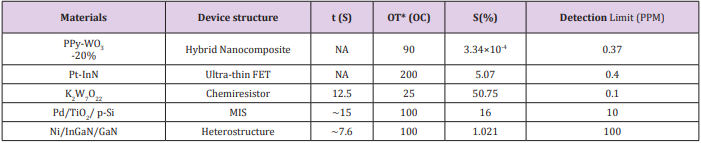
As a summary, Table 1 describe the sensing performances based above materials for application in acetone detection. Considering the application purpose - a tool of early-stage diabetes diagnosis, it requires that the sensor should have the detection limit as low as 0.9 ppm. As shown in Table 1, among these five materials, the detection limits of PPy- WO3 (20%), Pt-InN, and K2 W7 O22, are 0.37 ppm, 0.4 ppm, and 0.1 ppm respectively. The PPy-WO3 (20%) material-based sensor has the lowest detection limit of 0.37 ppm. However, its sensitivity is very small, only about 3.34 × 10-3 % with concentrations of acetone from 19 ppm to 316 ppm. So, the PPyWO3 (20%) material would not be a good option for breath acetone detection due to such poor sensitivity. The Ni/InGaN/GaN based Hetero-structure sensor (7.6 & 2 sec) shows a faster response and recovery time at 100 ppm of acetone as compared to Pd/TiO2 /p-Si MIS sensor (16 & 30 sec). However, a lowest detection limit of 100 ppm is far too high to detect the threshold of acetone, 0.9 ppm. The MIS sensor can get the detection limit down to 10 ppm but still too high to be used for the detection of breath acetone in diabetics. Due to these limitations, these two sensors cannot be a choice for breath acetone detection in diabetes diagnosis and monitoring. Considering the detection limit, within these materials, only Pt-InN and KWO do have the capability of detecting concentration of acetone less than 1.0 ppm with relatively good sensitivity.
Table 1: Centralization degree in North Africa and Sub-Saharan Africa, in terms of population distribution.
*OT: Operating Temperature
NA: Not Available
τ: response time
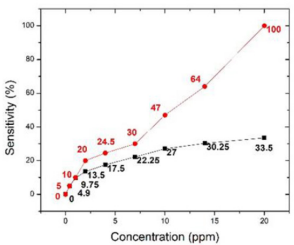
In Figure 7, we further compared the sensing response to acetone only focusing on the materials of KWO and the Pt-InN (This graph is based on data in Figures 4b [13] in black and 3a [19] in red). Figure 7 shows that the KWO based chemiresistive sensor demonstrates much higher sensitivity than the Pt-InN based sensor has, while acetone concentrations vary from 0 to 20 ppm. In addition, the KWO sensor can operate at room temperature without requiring any external source of heat, while Pt-InN sensor needs to work under 200o C. All these indicate that the nanostructured KWO based acetone sensor is an optimal device with less power consumption and higher sensitivity.
Figure 7: Gene ontology analysis of somatic mutations. (A) GO biological process classification; (B) GO cellular component classification; (C) GO molecular function classification.
Also, the KWO sensor device is simple and easy to be made [12] due to its chemiresistive sensing mechanism. The main reason why nanostructured K2 W7 O22 shows much better sensing performance on the detection of acetone can be explained by its high surface to volume ratio due to the nanoscale and porous structure and the specific material property - the room-temperature ferroelectric property of KWO (detailed discussion can be found in our published paper [24]). All of these properties result in an efficient surface interaction between KWO and acetone. While, the sensing processes based on the other four acetone sensors, all need to operate at an evaluated temperatures (> 100o C). This is mainly due to a surface oxidation reaction between the materials and acetone [30,31]. In a word, according to above discussion, it reveals that the material and structure properties play the most important role in detecting acetone. Since as-fabricated nanostructured KWO has a room-temperature ferroelectric property and high surface area [19,24], it provides an effective surface to sensitively interact with high dipole moment molecules such as acetone even at a low operating temperature.
Conclusion
We have studied and compared five different sensing materials with different operating conditions to detect acetone, the breath biomarker of diabetes. Within these materials, the nanostructured KWO showed the best response to acetone, which can detect the lowest concentration of acetone up to 0.1 ppm with fast response only around 12s at room temperature. As a completely newly synthesized nanostructured material, this unique material and structure property – room temperature ferroelectric property and high surface area with porous structure provide a favourable charge transfer and diffusion channel between KWO and acetone, which causes a strong interaction with acetone. In a word, KWO proves to be a promising material with great potential for diabetes diagnosis and monitoring under practical applications.
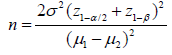

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.