Therapeutic Strategies of Kidney Transplant Ischemia Reperfusion Injury: Insight From Mouse Models
Introduction
End-stage renal disease (ESRD) is a global public health problem with generally poor outcomes and high costs. According to the latest U.S. Renal Data System Annual Data Report (2018), more than 720,000 Americans are being treated for ESRD. Kidney transplantation (KT) has become the optimal treatment for ESRD. However, all transplanted kidneys inevitably experience ischemia/ reperfusion injury (IRI) - the restriction of blood flow and the restoration of blood supply during transplant process trigger a cascade of noxious events, including the microvascular alteration, reactive oxygen species (ROS) responses, cytokine and chemokine release, and leukocyte activation, resulting in sterile inflammation and acute tubular necrosis (ATN) in renal grafts [1,2]. Severe transplant IRI frequently translates into delayed graft function (DGF), a common complication associated with a high morbidity and mortality post-KT and allograft rejection [1,3-5].
With a growing gap between kidney demand and supply, the quality of the donor kidney pool continues to deteriorate as the increasing donor age and associated comorbidities accumulate [6,7]. Expanded criteria donor kidneys of perceived lower quality are particularly susceptible to IRI, which compromises graft outcomes [7]. Therefore, seeking strategies to understand the pathophysiology of KT IRI and to develop effective treatments is highly desirable for transplant recipients [8]. In recent years, our laboratory and others have been attempting to adapt the mouse KT into a more clinically feasible model to investigate the mechanisms and new therapeutic approaches of transplant IRI. In this minireview, we discuss some of the lessons learned from mouse models of KT with regard to factors that influence the severity of transplant IRI and the potential therapeutic targets.
Clinically Relevant Mouse Models of Kidney Transplantation With Extended IRI
Mouse models of vascularized kidney transplantation have been widely used to dissect mechanism of IRI and transplant rejection. Similarly to the clinic, prolonged cold ischemic time (CIT) induce extended IRI in mouse renal grafts, thus introducing a clinically-relevant model for transplant IRI studies (Table 1) [9-17]. While the warm ischemic time (WIT) in most studies was limited to 30 mins, the CIT ranged from 20 mins to 8 hrs. In some studies, both of the native kidneys of recipient mice were removed during the transplant surgery, while in other studies one of the recipient’s native kidneys was kept in situ until day 4-5 post-transplant. The advantage of removing both native kidneys right away is that the immediate renal graft function will be accessible; however, it may also cause more mortalities during the early post-transplant phases due to the intensive IRI. Concerning the donor and recipient strains, both syngeneic and allogeneic mouse models have been employed for testing IRI treatments. However, since the contributions remain unclear with respect to the allogeneic immune responses versus donor/recipient genetics in graft IRI, the influence of mouse genetic background should be also taken into consideration when designing studies.
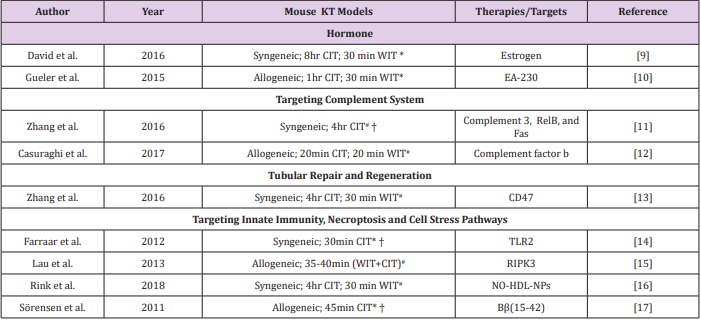
Table 1: Potential therapies or targets of kidney transplant IRI identified by utilizing the modified mouse models of KT with extended IRI.
Note: #Both native kidneys of recipient mice were removed during transplant surgery. *One native kidney of recipient mice was kept in situ to 4-5 days post-transplant. † Warm ischemic time was not reported. CIT: cold ischemic time; WIT: warm ischemic time; TLR: Toll-like receptor; RIPK: receptor-interacting protein kinase; NO-HDL-NPs: nitric oxide-delivering high-density lipoproteinlike nanoparticles.
Hormonal Influence
Sex disparities in kidney IRI tolerance have been proven in both animal systems and human studies [9,18]. By utilizing a mouse KT model with 8hr CIT, Aufhauser et al. [9] showed that female recipient mice had improved renal ischemia tolerance compared to male recipient mice, which correlated with better transplant outcomes. They found that renal IRI was exacerbated in female mice with estrogen receptor α deficiency, while female mice receiving supplemental estrogen prior to ischemia were protected [9]. Moreover, Gueler et al. [10] showed that the EA-230, an oligopeptide derived from β-human chorionic gonadotropin (β-hCG) lysates, ameliorated renal ischemic injury, improved renal allograft function, and prolonged survival in a mouse model of KT with 1hr CIT. These studies suggest the potential benefits of hormone supplements in kidney transplant IRI [10].
Complement System
Several experimental models of acute kidney injury (AKI) induced by clamping have demonstrated a clear-cut role for complement system in renal IRI [19,20] yet only a few studies have tested the effect of complement inhibitors in kidney transplant IRI models [12,21]. Using syngeneic mouse KT models with 4hr CIT, Zheng et al. showed that the administration of siRNA cocktail solution targeting complement 3, RelB, and Fas significantly reduced the expression of proinflammatory cytokines, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), decreased cell apoptosis, and improved renal function [11]. Recently, Casiraghi et al. [12] showed that BALB/c kidneys transplanted into complement factor b-deficient B6 recipients exhibited reduced IRI and diminished T cell-mediated rejection. The administration of anti-complement factor B antibody to recipients in early post-transplant phases ameliorated both IRI and early adaptive immune responses [12].
Tubular Repair and Regeneration
It has been accepted that proximal tubule regeneration postAKI occurs from intrinsic tubule cells [22]. Therefore, seeking strategies to promote tubular repair and regeneration holds promise for ameliorating renal graft damage. Our collaborative studies with Isenberg’s Lab showed that the treatment of renal tubular epithelial cells with a CD47 blocking antibody or CD47- targeting siRNA increased expression of self-renewal transcription factors and promoted cell proliferation [13]. Further studies in mouse models of KTwith 4hr CIT indicated that the treatment with a CD47 blocking antibody increased self-renewal transcription factor expression, decreased tissue damage, and improved renal function compared to that in control mice [13].
Targeting Innate Immunity, Necroptosis, and Cell Stress Pathways
It is known that IRI triggers a vast array of inflammatory mediators that activate innate immune responses. Toll-like receptors (TLRs) are critical molecules involved in inflammation. By using a mouse KT models with 1hr CIT, Farrar et al. showed that inhibition of TLR2 with a therapeutic agent (OPN301) significantly decreased acute tubular necrosis and improved renal graft function compared to controls [14]. Necroptosis, a term for programmed cell necrosis, is an emerging entity that might be involved in transplant IRI. This process is characterized by a pathway dependent on the receptor‐interacting protein kinase 1 (RIPK1)-RIPK3 complex [23]. A recent study by Lau et al. showed that BALB/c mice receiving RIPK3-/- kidneys had improved renal function and longer survival compared to controls, suggesting that inhibition of necroptosis in donor organs may provide a clinical benefit [15].
Other therapeutic targets testing in the mouse KT models with prolonged CIT included the nitric oxide-delivering highdensity lipoprotein-like nanoparticles (NO-HDL-NPs) and Bβ(15- 42), a breakdown product of fibrin that could inhibit leukocyteendothelial adhesion, as summarized in Table 1 and [16, 17]. Besides the therapeutic approaches summarized above, other potential targets that can be tested in the modified mouse models of KT include the mitochondrial dysfunction [24], endoplasmic reticulum stress [25], and lipid peroxidation [26]. With regard to the mouse model of KT IRI, the major limitation is that the surgical procedure is technically challenging and requires extensive microsurgical training. Few people in the world are able to perform this procedure, making it less convenient for translational research. Nevertheless, this model is very attractive as it is powerful and clinically-relevant for the mechanistic investigations and for testing new strategies, thereby offering a great opportunity for identifying new therapies for transplant IRI. Furthermore, compared to mouse AKI models in which no transplant procedure is included, mouse KT IRI model can efficiently test the mechanisms of kidney intrinsic factors (donor) versus extrinsic factors (recipient).
Conclusion
The progress of therapies in KT IRI relies on a better understanding the pathophysiology of renal injury and repair. Mouse models of KT combined with extended IRI could serve as a powerful tool for exploring the mechanisms of renal graft injury and for testing new therapies for transplant recipients.
More BJSTR Articles : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.