Isolation and Molecular Docking Studies of Dihydroimperialine as Butyrylcholinesterase Inhibitor from the Bulbs of Fritillaria Imperialis
Acetylcholinesterase is an enzyme whose primary function is to breakdown of acetylcholine into choline and Acetyl-CoA, which effects on the memory loss and other intellectual properties. The Alzheimer’s Disease (AD) is associated with the loss of cholinergic neurons in the brain and the decreased level of ACh [1,2]. Fritillaria imperialis L commonly known as “crown imperial”. The plant usually grows in rocky slopes and amongst scrub at attitude of 1000-3000 meters in Turkey and other parts of eastern Europe. The bulb is known to be poisonous and contains low concentrations of toxic alkaloids. The genus Fritillaria belongs to the family liliaceae, which is an important steroid alkaloid producing plant family [3]. Up to now, more than 160 alkaloids have been reported from the genus Fritillaria. A few species of Fritillaria are native to Cyprus, Southern Turkey and Iran [4]. Among them F. roylei or F. verticillate (thembergii) commonly known as “Bei-mu” or “Pei-mu” in Chinese and “Bai-mo” in Japanese, has long been known as one of the principal Chinese crude drugs [3,5] having many therapeutic applications [6]. Many species of Fritillaria are traditionally used as herbal remedies in Japan, Turkey and south Asia [7]. F. camsehatcensis [8] is another species grows in northern Japan and is an important ingredient in the diet of Hokkaido (Japan). It has also been used as antitussive, expectorant, hypotensive and for antitumor activities [6,9,10].
There are several species of Fritillaria F. thunbergii, F. cirrhosa, F. persica, F. przewalski, F. Assuriensis, F. pallidiflora, F. delavay and F. maximowiczii used in indigenous medicine [11]. Several alkaloids, such as forticine, impericine, impranine, dihydroimpranine, fetisinine have already been reported from this plant, which showed acetylcholinesterase inhibitory activity [7,12-14]. Butyrylcholinesterase (E.C. 3.1.1.8), metabolizes cocaine to the water-soluble inactive compounds ecgonine methyl ester and benzoic acid [15]. The normal level of BChE varies among individuals and is dependent on age, state of health, exposure to environmental toxins and genetic factors. Low levels of BChE are associated with cocaine-induced cardiac and related complications [16]. Furthermore, deficiency of BChE shifts the metabolism of cocaine to norcocaine, a metabolite with anesthetic and cardiotoxic properties and benzoylecgonine, a metabolite that causes vasoconstriction [16,17]. BChE is distinguished from other esterases by a high affinity for cholinesterase and their thio analogs [17]. It has also been found that butyrylcholinesterase inhibition may also be an effective tool for the treatment of AD and related dementias [17]. Availability of the X-ray crystal structure of butyrylcholinesterase with butyrylcholine (PDBcode: 1P0P) prompted us to perform molecular docking studies. To further explore the binding mechanism of compound 1, molecular docking studies were carried out by automated GOLD docking software.
Materials and Methods
General
Optical rotations were measured on a Jasco DIP-360 digital polarimeter by using 10 cm cell tube. IR spectra were recorded on a Jasco A-302 IR spectrophotometer. IH-NMR spectra were recorded on Bruker 500 MHz NMR spectrometer and chemical shifts were calculated with reference to CDCl3 (7.25). 13C-NMR spectra were recorded at 125MHz on a Bruker AM 500MHz NMR spectrometer. Mass spectra were recorded on a Varian MAT 312 double focusing spectrometer, connected to an IBM-AT compatible PC computer system. Column chromatography was performed on silica gel (70-270 mesh, ASTM, Merck) and flash silica gel (230-400 mesh, ASTM, Merck). TLC was done on percolated TLC cards (Merck) with solvent system of acetone: pet ether: diethylamine (3:6.5:0.5).
Plant Material
The plant material was already explained in our previous publications [12,13]. The extraction and isolation procedure is the same as described in our previous publication [13]. Column chromatography of the subfraction A (1.20 gm) afforded another subfraction B (205.6 mg). Further flash column chromatography of the subfraction B afforded the semi pure compound C (37.9 mg). Semipure compound 1 was finally purified (16.4 mg) by flash column chromatography including compound 2 (9.0 mg) by using acetone-hexane mixture containing a few drops of diethylamine. The physical and spectroscopic data of compound 1 are given below:
Dihydroimperialine (1): Amorphous powder from chloroform (16.4 mg), []D 25 = -16° (c = 0.5, CHCl3 ). IR (CHCl3 ) υmax cm-1: 3415-3409 (OH stretching), 2975 (18), 2896 (-CH stretching). UVλmax (MeOH) nm (logε): 351 (2.19), 222 (3.49), 208 (3.77). MS: m/z 431. HREI MS: m/z (mol. formula, calcd. value): 431.4226 (calcd 431.4228 for C27H45NO3 ). MS (EI, 70 eV): m/z (rel. int %): 431[M+ ] (40), 415 (16), 414 (26), 400 (23), 388 (8), 374 (24), 234 (10), 112 (100), 98 (88). 1 H-NMR (CDCl3 , 400 MHz) 1.01 (3H, s, H-19), 1.08 (3H, d, J 27, 25 = 7.0 Hz, H-27) 1.09 (3 H, s, H-21), 3.54 (1 H, br, m, W1/2 = 25.0 Hz, H-3), 3. 80 (1 H, br, d, W1/2 = 8.0 Hz, H-6 α). 13C-NMR (125, MHz): 14.6 (C-19), 14.7 (C-27), 17.9 (C-21), 18.8 (C-16), 20.2 (C-20), 27.8 (C-25), 28.4 (C-15), 28.5 (C-24), 29.5 (C2), 31.3 (C-11), 34.6 (C-4), 39.3 (C-7), 39.4 (C-1), 39.6 (C-8), 40.9 (C-14), 41.8 (C-13), 43.8 (C-17), 48.1 (C-5), 57.7 (C-9), 59.6 (C-18), 61.3 (C-26), 63.6 (C-22), 70.9 (C-20), 71.9 (C-3), 73.3 (C-6).
Cholinesterase Inhibition Assay
The AChE and BChE inhibiting activities were measured by spectrophotometric method developed by Ellman [18]. Electric-eel AChE (EC 3.1.1.7), horse-serum BChE (E.C 3.1.1.8), acetylthiocholine iodide, butyrylthiocholine chloride, 5, 5´-dithiobis (2–nitrobenzoic acid) (DTNB), and galanthamine were purchased from Sigma (St. Louis, MO, USA). All the other chemicals were of analytical grade. Standard operational assay protocol has been described previously [19]. All the kinetic experiments were performed in 96-well microtitre-plates by using Spectra Max 340 (Molecular Devices, CA, USA). The rate of the enzymatic reaction [18] was measured by the following equation.
Determination of IC50 Values
The concentration of compounds that inhibited the hydrolysis of substrates (acetylthiocholine and butyrylthiocholine) by 50% (IC50) was determined by monitoring the effect of various concentrations of the compound in the assays on the inhibition values. The IC50 values (inhibitor conc. that inhibits 50% activity of AChE and BChE) were then calculated using the EZ-Fit Enzyme Kinetics program (Perrella Scientific Inc., Amherst, USA).
Molecular Docking Methods
Pretreatment of Protein. The high-resolution crystal structure of butyrylcholinesterase in complex with butyrylcholine was obtained from the Protein Data Bank (PDB ID: 1P0P) [20]. All hydrogens were first added into the PDB using the SYBYL program package, version 6.9 [21]. Protonation states were set according to the program, based on the likelihood that the residues would be charged at physiological pH, together with their chances to form hydrogen bonds with the surrounding environment. All water coordinates and metal were removed from the protein file. Important residues, especially in the binding site were visually inspected and corrected according to charge and according to atom and bond type, especially the pH of lysines, arginines, aspartates and glutamates.
Pretreatment of Ligand
The ligand that was selected for the docking studies was drawn using the sketch module of SYBYL. Ligand was inspected and refined to correct stereochemistry and atom and bond type according to GOLD specifications. In order to obtain lowest energy conformation of the ligand, the overall geometry optimization was carried out by Powell method [22] using Tripos force field with a convergence criterion of 0.05 kcal/mol. Å by giving 1,000 iterations. Charge distributions were calculated by the Gasteiger-Marsili method [23].
Docking in GOLD
The GOLD software (Version 3.0) [24] was used to dock the compound onto the active site of BChE in order to determine the probable binding conformation of inhibitor. The scoring of the dockings was based on the scoring functions implemented in the programs. In the Gold runs, the Gold score scoring function was used. The search area in the docking runs was limited to include the cavity that forms the active site of the protein and parts of the so-called hydrophobic pocket adjacent to the active site region, which is docking region and enclosed by a sphere of 10 Å centered on the center of gravity of the butyrylcholine (experimental ligand). In each case, 100 conformations of the ligand were produced and clustered by similarity. Poses were evaluated using Gold score. To test the reliability of the poses generated by GOLD, the ability to reproduce the experimental binding mode of butyrylcholine was tested. The experimental ligand was taken out of the X-ray structure, and a new conformation of butyrylcholine was docked back into the binding site using the settings above. An average rmsd value of 1.5 Å between the 10 calculated poses and the experimental pose indicates a reliable prediction of the binding mode. Threedimensional structure building and all modeling were performed using on Intel® Xenon® Quad™ core processor running under opens USE 11.0 LINUX environment.
Results
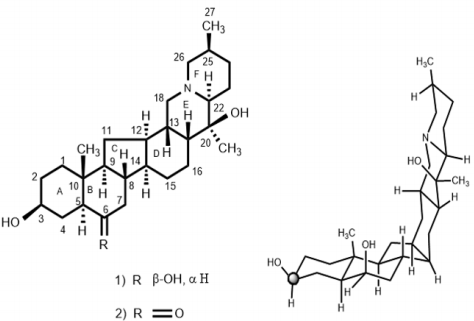
The chloroform fraction was eluted from the column chromatography with hexane-acetone (65:35%) mixtures and compound 1 was isolated as a colorless powder. The IR spectrum of compound 1 showed strong absorption bands between 3409-3415 cm-1 for the hydroxyl group and at 2975 cm-1 for trans-quinolizidine moiety [25]. The EI-MS of compound 1 exhibited the [M+ ] at m/z 431.4226, in agreement with the formula C27H45NO3 (calcd. 431.4228), suggesting six degrees of unsaturation in the molecule. The mass spectrum of compound 1 also showed important fragments at m/z 431, 415, 414, 388, 386, 374, 234, 112 and 98. The base peak at m/z 112 resulted from the cleavage of the C-20/C-22 and C-13/C-18 bonds, suggesting the presence of a heteroatom at the junction of rings E and F [26]. The 1 H-NMR spectrum (CDCl3 , 500 MHz) of compound 1 exhibited signals for three methyl groups. Two 3H singlets, resonating at δ 1.01 and 1.09, were assigned to the C-19 and C-21 methyl protons, respectively. A 3H doublet at δ 1.08 (J27, 25 = 7.0 Hz) was assigned to the C-27 methyl protons. Two downfield signals appearing at δ 3.54 (br., m, W1/2 = 24.0 Hz) and 3.80 (W1/2 = 8.2 Hz) were assigned to the C-3 and C-6 protons, respectively [12,13]. The spectral data of compound 1 was identical to the earlier reported data of dihydroimperialine a reduced derivative of imperialine (2) [27,28]. The 13C-NMR (CDCl3 , 100 MHz, BB and DEPT, Table 1) spectra showed signals for all 27 carbon atoms. Two downfield signals resonating at 71.9 and 73.3 were assigned to the OH-containing C-3 and C-6 carbons atoms, respectively. Two quaternary carbons at 35.5 and 70.9 were assigned to the C-10 and C-20 carbon atoms, respectively (Figure 1).
The stereochemistry at the asymmetric center was elucidated on the basis of the 1H-NMR and 13C-NMR chemical shifts comparisons with imperialine (2). For the direct comparison of dihydroimperialine (1), known compound imperialine (2) was reduced with NaBH4 to obtain dihydroimperialine and compared with the naturally isolated dihydroimperialine (1). The mass spectra of compound 1 exhibited the ions at m/z at 431, 416, 415, 400, 386, 387, 234, 180, 155,154, 124 112, 98, and 55, like the mass fragmentation patterns reported for found in imperialine (2), zygacine and eduradine [29]. The base peak at m/z 112 and 98 indicated the presence of cevanine-type steroidal alkaloids [29]. Dihydroimperialine (1) was obtained as an amorphous colorless powder by column chromatography of the chloroform extract of F. imperialis and identified as (20S, 22S, 25S)-5α-cevanin-3β,6β-diol.
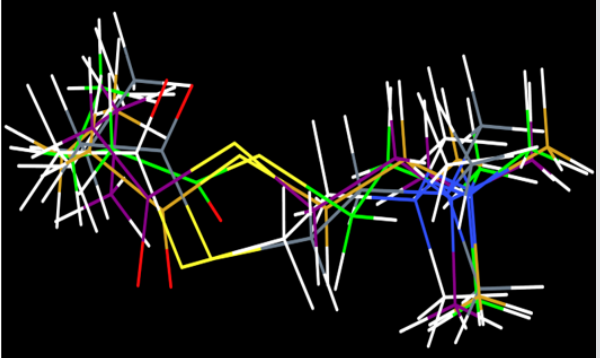
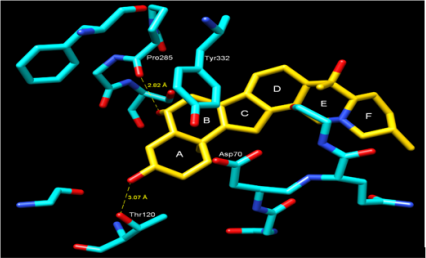
For molecular docking studies, initially to test the validity of Gold docking program, at the first stage the crystal structure of the BChE-Butyrylcholine complex (PDB ID: IP0P) was successfully reproduced. The root-mean-square deviation (RMSD) of the docked confirmation to the experimental confirmation was around 1.0 Å, Figure 2 suggesting a high reliability of GOLD in reproducing the experimentally observed binding mode of buytrylcholinesterase. The quality level of the docking complex was similar to that of the original crystal structure. Additionally, all top rank conformations were clustered at the active site with almost identical confirmations see Figure 2. This result suggested that the protocol applied for the docking simulation was sufficient for further analysis and could be accepted for virtual screening purposes in our study. Dihydroimperialine (1) was successfully docked on the active site of BChE keeping the same docking protocol used for reference ligand (Figure 3). Dihyroimperialine (1), which has a rigid structure, comprises six rings with one hydrogen bond accepter group at ring E and three hydrogen bond donar groups at ring A, B and E. The rigid steroidal backbone of compound 1 enters the aromatic gorge through the six-membered ring A, and thus, placed the ring A in the bottom of the gorge near Thr120.
Figure 2: Evaluation Of GOLD docking software; superimposition of top 4 poses showed almost the same bonding pattern as that of butyrylcholine.
Figure 3: Docking of compound Dihydroimperialine (1) and BChE, showing the hydrogen bonding, cation-π and π-π interactions. Amino acid residues in numbered are dominant residues that are involved in interactions.
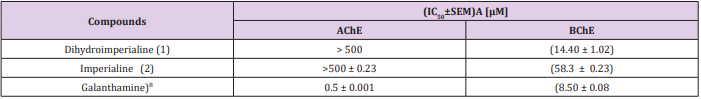
The ring A was stabilized by hydrogen bonding between hydroxyl group A and terminal hydroxyl group of Thr120 (3.07 Å) [30]. Another much stronger hydrogen bond was observed between the hydroxyl group B and the peptidic carbonyl atom of Pro285 (2.82 Å) (Figure 3). Additionally, the center of ring A into gorge of BChE is stabilized by cation– interaction contributed by Asp70. The ring B forms – interaction to one of the aromatic amino acid residues of gorge Tyr332. Compound 1 was evaluated for its butyrylcholinesterase inhibitory activity and found to be active against the butyrylcholinesterase enzyme with IC50 14.4±1.0 (μM). The inhibitory activity and IC50 values are given in Table 1. Conclusion: This is the first report of the isolation of dihydroimperialine (1) form natural source and molecular docking studies showed the sign for future drug discovery for Alzheimer’s disease.
Crown Height and its Relation to Arch Width, Arch Length and Arch Perimeter in Ideal Occlusion-https://biomedres01.blogspot.com/2020/10/crown-height-and-its-relation-to-arch.html
More BJSTR Articles : https://biomedres01.blogspot.com

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.