Perineural Migration of Stem Cells in the Model of Damaged Heart Nodes
Introduction
Issues related to arrhythmias and heart failure development still remain unresolved and socially important in all countries. This is confirmed by statistical data on high risk of mortality due to cardiovascular pathology [1,2]. Considering scientific literature [1,2], authors paid attention to reasonability of stem cells (SCs) use as additional therapy in heart diseases. Authors experimentally substantiated technology [3] based on the use of SC perineural migration along vagus nerve fibers to heart [3]. The fact of perineural migration of SC to damaged heart areas was experimentally proved on rats [3]. Destructive events in heart atriums and ventricles are caused by the process of degeneration of vagus and sympathetic nerve fibers. These neurodegenerative processes developed after transection of vagosympathetic trunk at cervical level in experiments on laboratory animals (Figure 1). Initiation of experimental destruction in heart [3] was required to test hypothesis on the ability of SCs to move along vagus nerve namely to damaged heart regions. Similar SCs ability was previously demonstrated in relation to brain [4-9]. It is known that SCs are necessary for reparation of damaged tissues. Recovery of cell and tissue structure with SCs is accompanied with normalization of functional activity of cells, organs and tissues.
Figure 1: Scheme of unilateral vagotomy and perineural implantation of stem cells. Explained in text.
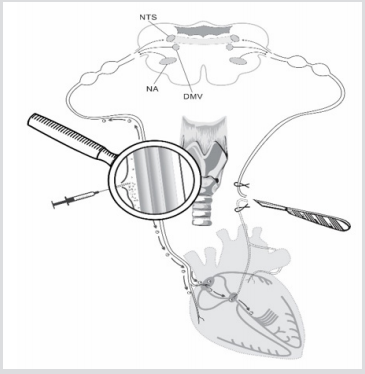
The mechanism of SCs migration to damaged areas is initiated by plenty of trophic factors and other signaling molecules appearing there. Unilateral vagotomy was chosen among various destruction models to minimize heart injuries (Figure 1). It was decided to inject SCs into perineural space of intact vagus nerve on the other side to exclude influence of neurotrophic factors on migration process. We supposed that SCs will start moving along vagus nerve fibers directly to those signaling molecules which will appear in the areas of degenerated vagus nerve fibers after its transection (Figure 1). Such destructive processes in heart are associated with the ones in real life which develop in patients with infarction, syncope events, vasovagal conditions and various arrythmias. Authors are interested, it is really and safely to use minimally invasive approach to inject SCs into perineural space of vagus nerve to activate reparative processes in automaticity nodes and those destructed heart regions which usually initiate development of cardiac arrhythmias and heart failure in patients. Authors understand that such technique of SCs perineural migration to heart could be implemented and used in complex classic therapy after approval by physicians
Vagus Nerve as One of the Ways of Stem Cells Delivery to Heart
Establishment of fact of SCs perineural migration to heart [3] was a kind of departure point to conduct series of experiments to reveal features of structural changes in heart which are the trigger for initiation of SCs migration processes to damaged tissue regions. 4 adult male Wistar rats weighing 190-220g at the time of the experiments have been chosen. Vagotomy (4 rats) was performed under ketamine-xylazine-acepromazine anesthesia (55.6, 6.6 and 1.1mg/kg, respectively, i/p). Unilateral (left side) vagotomy was performed in all four rats at the cervical level of neck (Figure 1). Two rats of four received 10 thousand SCs in 10µl culture medium in perineural space of vagus nerve after vagotomy (Figure 1). SCs were marked with monoclonal antibodies to CD90 and PKH67 Green Fluorescent Cell Linker
Rats were decapitated in one week after vagotomy and horizontal serial heart slices 8µm in thickness were prepared using Microm HM525 (Germany) cryostat. SCs distribution was visualized using Zeiss AxioVert 200M fluorescence microscope with Zeiss AxioCam HRm CCD camera. Ten serial heart slices were taken with the interval of 50µm, were stained in hematoxylin-eosin and structural changes were visualized using Fluorescent microscope MPV-2 (Leitz, Germany) with camera Leica DC 300F (Germany).
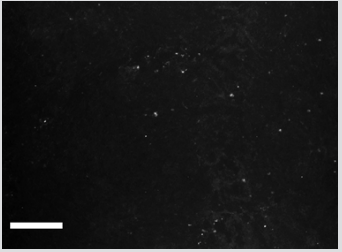
Figure 2 shows distribution of PKH67 Green Fluorescent Cell Linker labeled SCs in the area of left ventricle. SCs revealed in destructed region of heart ventricle in one week after injection into perineural space of vagus nerve prove that they moved there from the place of injection. Literature data on the vast innervation of heart ventricles by vagus nerve [10-18] confirms this point of view.
Figure 2: Distribution of PKH67-labeled SCs in the left ventricular heart of rat. Bar - 100μm. Explained in text.
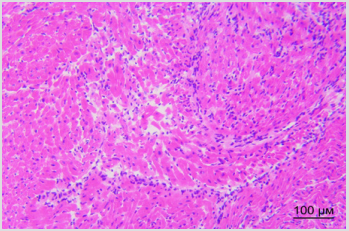
By the way, we established earlier that implanted SCs appear in sinoatrial heart node within the first day after vagus transection [3]. Figure 3A shows destruction foci in sinoatrial node in right atrium in rat in one week after vagus nerve transection. Figure 3B shows different picture: cells of sinoatrial node are visualized in right atrium of rat in one week after vagus nerve transection and perineural injection of SCs in intact vagus nerve. There were no signs of destruction noted in sinoatrial node in this case. Therefore, SCs migration along vagus nerve fibers to heart tissue is the basis for realization of reparative potential of SCs in the area of main pacemaker and such obvious way of SCs delivery to heart appears to be promising method of correction of structural and functional state of heart nodes and myocytes.
Figure 3A: Distribution of PKH67-labeled SCs in the left ventricular heart of rat. Bar - 100μm. Explained in text.
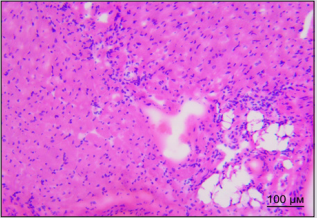
Figure 3B: Distribution of PKH67-labeled SCs in the left ventricular heart of rat. Bar - 100μm. Explained in text.
Characteristics of Cardiac Activity After Unilateral Vagotomy at Cervical Level
Separate series of experiments was performed on 11 Wistar rats weighing 190-220g at the time of the experiments. Heart rate was analyzed with non-invasive pulse recorder L120E INSELT (USA) before unilateral vagotomy on different sides and repeatedly after vagotomy. Surgical procedures were performed as described above. Four animals were subjected to unilateral vagotomy alone. Seven animals received vagotomy together with simultaneous injection of SCs into intact vagus nerve. All the rats showed heart rate changes and intermittent arrhythmia regardless the side of manipulation in one day after unilateral vagotomy: bradycardia in 7 rats, tachycardia in 4 rats. Heart rate was not recovered in those rats who didn’t receive SCs injection even in one week after vagotomy; arrhythmia worsened in one rat. Heart rate recovered in all rats received SCs injection together with unilateral vagotomy.
Conclusion
Protective role of perineural SCs injection in relation to destructive processes development in heart nodes was demonstrated in experiments on Wistar rats using unilateral vagotomy technique. Preservation of sinoatrial node cells structure and recovery of cardiac rhythm was established after unilateral vagotomy and injection of SCs into intact vagus nerve. Therefore, method of SCs perineural migration along vagus nerve fibers to heart seems promising for prevention of arrhythmias and heart failure development in patients with impaired cardiac function of organic origin.
Paget’s Disease of the Nipple Presenting as Non-Healing Ulcerative Lesion of Nipple: A Case Report and Review of Literature-https://biomedres01.blogspot.com/2021/03/pagets-disease-of-nipple-presenting-as.html
More BJSTR Articles : https://biomedres01.blogspot.com

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.