High Intermediate Risk Pulmonary Embolism: The Use of Very Low Dose Catheter-Directed Ultrasound- Accelerated Thrombolysis
Introduction
Outcomes in acute pulmonary embolism (PE) vary substantially
depending on the presences or absence of hypotension [1]. In
massive, High-Risk PE complicated by cardiogenic shock or the need
for cardiopulmonary resuscitation, in-hospital mortality rates are
25% and 65%, respectively [2,3]. Intermediate Risk PE represents a
group characterized by the presence of right ventricular dysfunction
(RVD) without systemic hypotension [2,4]. Estimates of in-hospital
mortality for such patients have been as low as 2-3% as reported
in a recent multicenter trial [5]. Despite the overall low mortality
rate, 5-25% will demonstrate hemodynamic and clinical worsening
requiring hemodynamic support and/or rescue thrombolysis [5,6].
Substantial variability exists in clinical outcomes in Intermediate
Risk PE. In patients with RVD on echocardiogram combined with
elevated troponin I mortality rates have been reported between
8-15% [7,8]. Patients with RVD and concomitant lower extremity
deep venous thrombosis (DVT) have a PE-related mortality of
nearly 20%, approximating that of massive, High-Risk PE [7].
In addition to mortality, persistent RVD and the development of
chronic thromboembolic pulmonary hypertension (CTEPH) is
substantially higher following a submassive Intermediate risk PE
[9-12].
Patients who have an initial pulmonary artery systolic pressure
over 50 mmHg are at significant risk [13,14]. While systemic
thrombolysis has been shown to reduce mortality in High-Risk
PE [15], use in Intermediate Risk PE has been limited due to
major bleeding complications, including intracranial hemorrhage
(ICH) [5]. Recently, catheter-directed ultrasound-accelerated
thrombolysis (USAT) has been developed as a treatment option for
Intermediate Risk PE [4,16-24]. USAT has been shown to rapidly
reverse pulmonary hypertension (PH) and RVD by significantly
reducing pulmonary clot burden without major bleeding
complications [4,16-24]. Additionally, there is increasing evidence
that early clot reduction improves hemodynamic parameters
and reduces persistent RVD [9,18,19]. Despite increasing clinical
experience with USAT, variability exists in thrombolytic dosing and
duration of therapy [4,16-24]. The purpose of this retrospective,
single-center study, is to further describe the use of USAT, utilizing
a lower dose thrombolytic protocol than previously reported and
its impact on pulmonary artery pressure, clot burden and safety in
high Intermediate Risk PE. In addition, we define high Intermediate
Risk PE as those at greatest risk for hemodynamic deterioration,
death, or the development of CTEPH.
Material and Methods
Study Design and Patients
A retrospective study of patients admitted with high
Intermediate-Risk PE treated with USAT between July 2012 and
February 2016 was performed. The study was conducted at a
single academic center with approval from the Lankenau Medical
institutional review board. (IRB# F/N-R14-3414L). During the
study period, 52 patients (27 males) were treated with USAT
utilizing the EkoSonic Endovascular System (EKOS®) (EKOS
Corporation, Bothell, Washington, United States of America) with
recombinant tissue plasminogen activator (r-tPA) and concurrent
intravenous (IV) unfractionated heparin infusion. Informed
consent was obtained in all patients prior to undergoing USAT. PE
was diagnosed in all patients by computerized tomography (CT)
angiography with symptom onset < 2 weeks. Intermediate Risk PE
was defined as the presence of RVD without systemic hypotension
[2,4]. RVD was detected utilizing electrocardiogram, cardiac
biomarkers, CT scan, and transthoracic echocardiography.
High Intermediate Risk PE was considered those at greatest
risk for acute hemodynamic deterioration, death or subsequent
development of CTEPH (Table 1). All Patients had Right ventricular
dilation on CT (Right Ventricular/Left Ventricular (RV/LV)
maximum diameter ratio >0.9 on standard axial views. In addition,
by our protocol, patients had elevation of troponin I (>0.05 ng/
mL) and/or B-type natiuretic peptide (>90pg/ml) and a proximal
DVT or estimated right ventricular systolic pressure (RVSP) on
echocardiogram >50 mmHg. Lower extremity Doppler ultrasound
was performed in the majority of patients to exclude deep venous
thrombosis (DVT). If lower extremity DVT was present, retrievable
inferior vena cava (IVC) filter was placed at the discretion of the
treating physician. Patients were deemed candidates for USAT if
they met clinical criteria for acute high Intermediate risk PE and had
evidence of thrombus within the main or lobar pulmonary arteries
on CT. The Department of Interventional Radiology performed
USAT. All patients were admitted to the medical intensive care unit
upon diagnosis and during USAT treatment.
Table 1: Clinical Criteria Characterizing High Intermediate Risk Pulmonary Embolism.
Note: Definition of abbreviations used: RV dilation = right ventricular/left ventricular (RV/LV) diameter ratio > 0.9 on echocardiogram or CT scan; DVT = deep venous thrombosis. Cardiac biomarkers = troponin I and/or B-type natiuretic peptide; RVSP = right ventricular systolic pressure; CTEPH = chronic thromboembolic pulmonary hypertension.
Standardized Procedure of Catheter-Directed Ultrasound-Accelerated Thrombolysis
All patients were treated with the use of EkoSonic MACH4e Endovascular System (EKOS®). The EKOS® system consists of a removable microsonic device that transmits microsonic energy into the clot. The insertion of the EkoSonic Endovascular System was performed under aseptic technique with continuous cardiac monitoring. Following central venous access, IVC venography was performed to ensure no thrombosis was present. IVC venography was incorporated in our protocol in 2015. Subsequently, left, right and main pulmonary arterial pressures were obtained. Selective left and right pulmonary arteriography was performed to determine clot burden and pulmonary arterial perfusion. The main and lower lobe pulmonary arteries were considered for catheter insertion only. Bilateral device placement was performed when emboli were located in both main or proximal lower lobe arteries. A continuous infusion of r-tPA was then initiated at 0.5mg/hr through each catheter for bilateral treatments (1.0mg/hr for unilateral catheters). After successful device placement, patients were transferred to the medical intensive care unit for continuous monitoring. Treatment was continued for up to 24 hours, at which time the EKOS® devices were exchanged for angiographic catheters, and repeat pulmonary arteriography was performed in the interventional radiology suite to reassess clot burden, pulmonary arterial perfusion, and pulmonary arterial pressures. The introducer sheath(s) were removed, and hemostasis was obtained by manual compression.
Anticoagulation Therapy
IV unfractionated heparin was utilized in all patients prior to, during, and immediately following USAT. Prior to undergoing USAT, unfractionated heparin was administered utilizing a standard weight-based nomogram. Activated partial thromboplastin time (aPTT) was monitored with a therapeutic target range of 68-101 seconds. During USAT, a low dose unfractionated heparin protocol was instituted. The rate of heparin infusion was adjusted to achieve and maintain an aPTT between 40-60 seconds. Following catheter removal, the standard weight-based nomogram was resumed, targeting an aPTT of 68-101 seconds. Subsequent anticoagulation was left to the discretion of the treating physician.
Primary Outcomes
The primary outcomes assessed in this study were the change in pulmonary artery pressure and extent of angiographic reduction in clot burden utilizing the Miller score [25] immediately following USAT. Pulmonary arterial pressures were obtained at the time of catheter insertion and catheter removal. Miller scores were determined from the pulmonary angiograms performed at the time of catheter insertion and catheter removal. Two diagnostic radiologists interpreted and scored the pulmonary angiograms independently. The Miller scores were averaged for the analysis.
Secondary Outcomes
The secondary outcomes assessed included bleeding, procedural related complications and all cause in-hospital mortality. Major bleeding complications were defined as ICH or bleeding severe enough to warrant cessation of therapy or blood product transfusion. Minor bleeding complications were defined as bleeding manageable with local compression, sheath upsizing, or unfractionated heparin and/or thrombolytic dose reduction [8]. Bleeding complications were assessed during USAT and up to 72 hours following catheter removal.
Statistical Analysis
Paired t-test was performed to test the hypotheses that the pre-USAT mean pulmonary artery pressure, pre-USAT pulmonary artery systolic pressure and pre-USAT Miller score were equal to the post-USAT mean pulmonary artery pressure, post-USAT pulmonary artery systolic pressure and post-USAT Miller score, respectively. The normality assumption for performing paired t-test was satisfied for all three comparisons by using kurtosis, skewness and shapiro-wilk test.
Results
Clinical Characteristics
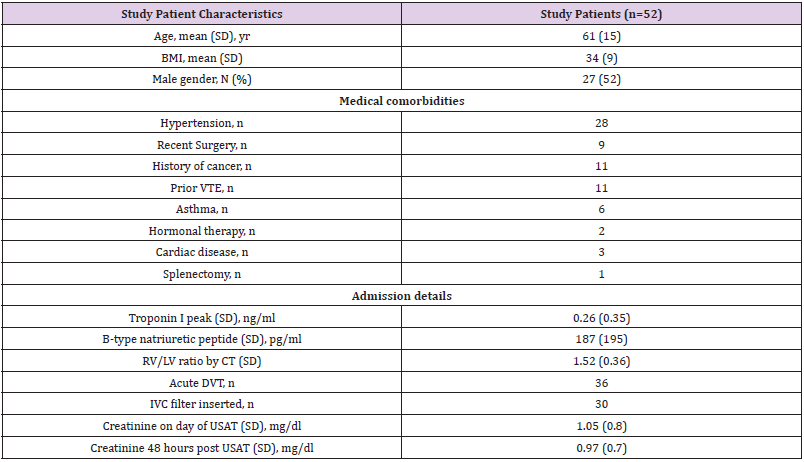
During the study period, 52 patients (27 males) underwent USAT for high Intermediate Risk PE. Patient characteristics are outlined in Table 2. 30 patients were unprovoked PE and 9 were provoked post surgeries and 11 patients had a diagnosis of cancer and 2 were on hormonal therapy. 11 had a history of prior VTE. The mean RV/ LV ratio on the CT was 1.52 + 0.36. 48 patients had elevated cardiac biomarkers (Troponin I and/or B type Natiuretic Peptide). The 4 patients who did not were treated because of elevated PASP>50 and +DVT. 38 patients had initial RVSP>50 on initial echocardiogram. Lower extremity DVT was identified in 36 patients on the day of diagnosis by Ultrasound. Of these, 30 patients had a retrievable IVC filter placed. 30 patients met all 3 criteria listed in Table 1, and only 2 patients met 1 criteria (+troponin, criteria #2).
Procedural Details
Bilateral catheters were utilized in 49 of 52 patients. Recombinant t-PA was infused at 0.5mg/hour/catheter in patients undergoing bilateral USAT (1.0mg/hour with unilateral USAT). The average r-tPA dose in our cohort was 22.1 + 4.9mg. The average duration of USAT was 22.1 + 4.9 hours. All patients received concurrent IV unfractionated heparin. The mean aPTT during thrombolytic infusion was 50.0 + 13.9 seconds. Goal aPTT (40- 60 seconds) during thrombolytic infusion was achieved in 58 % (n=30/52) patients.
Change in Pulmonary Arterial Pressures
Overall, there was a statistically significant reduction in the pulmonary artery systolic pressure and mean pulmonary artery pressure immediately following USAT. The pulmonary artery systolic pressure decreased 7.3% from 63.6 + 15.1mmHg to 59.0 + 17.5mmHg (p value = 0.0045). The mean pulmonary artery pressure decreased 5.4% from 37.5 + 8.0 mmHg to 35.5 + 9.1mmHg (p value = 0.0097). Thirty-eight patients had a PASP > 50 mmHg at the time of initial catheterization. Within this subgroup, a 6.2% reduction in PASP was observed following USAT (67.0 + 14.0 mmHg to 62.9 + 16.6 mmHg; p value = 0.0023). Only 7 patients had reductions in PASP < 50 mmHg immediately following USAT despite a 53.1% reduction in Miller score (20.0 + 3.9 to 9.4 + 4.7; p value <0.0001).
Change in Miller Score
Miller scores were computed for 50 of 52 patients. One patient had a non-diagnostic pulmonary arteriography and another had imaging via carbon dioxide digital angiography, therefore she was excluded. There was a statistically significant decrease in the Miller score immediately following USAT. The Miller score decreased 55.0% (mean 19.7 + 3.8 to 8.9 + 4.7; p value < 0.0001).
Safety
No procedural related complications occurred. There were no major or minor bleeding complications. No patient received blood transfusions.
Mortality
The in-hospital mortality rate for this cohort was 1.9% (n=1/52). The death was not procedural related. The patient underwent bilateral USAT. Despite a 52.9% reduction in Miller score, pulmonary arterial pressures worsened (PASP 73 mm Hg to 78 mm Hg; mean PAP 38 mm Hg to 44 mm Hg). Three days after USAT, the patient suffered an acute neurological event. Emergent CT imaging revealed new pulmonary embolism with evidence of occlusive thrombus within the aortic arch extending into the proximal descending aorta, right and left common carotid, left middle cerebral, left subclavian, and left vertebral arteries. Lower extremity ultrasound from admission had revealed an acute popliteal DVT. IVC filter had not been placed. Transthoracic echocardiogram on the day of neurologic deterioration demonstrated a patent foramen ovale (PFO) and a non-occlusive thrombus within the IVC. Unfortunately, the patient progressed to brain death.
Discussion
Intermediate Risk PE accounts for over 30% of all hospitalized
PE [26]. The in-hospital mortality rate in such patients is estimated
at 2-3% based on contemporary studies [5]. Despite the overall low
mortality rate, there is substantial variability in clinical outcomes
with some at risk for hemodynamic deterioration and some at
risk for the development of CTEPH. To accept the bleeding risk of
thrombolytic therapy, additional risk stratification is required. In this
study, we defined high Intermediate Risk PE as the subset of patients
that presented with acute symptoms and had CT angiographic evidence of clot within the central pulmonary circulation. These
patients additionally demonstrated the combination of RV dilation
(RV/LV>1.0 on CT scan) and elevated cardiac biomarkers (Table
1) with the additional findings of either proximal DVT or elevated
RVSP estimated on cardiac echocardiogram. Systemic thrombolytic
therapy, while beneficial in massive, High-Risk PE [15], has been
associated with major bleeding complications in Intermediate Risk
PE, including intracranial hemorrhage [5].
USAT is an attractive treatment strategy for Intermediate Risk PE
because it has the ability to rapidly reduce clot burden with a much
greater safety profile than systemic thrombolysis [4,16-24]. Studies
have consistently shown a reduction in RV/LV ratio [4,17,21-24]
pulmonary arterial pressure [4,16,18-24] and pulmonary arterial
clot burden [4,17,21-24] immediately following USAT. The recently
published PERFECT registry [21] has both confirmed the safety
of catheter directed therapies as well as demonstrating similar
reductions in Pulmonary artery systolic pressure. Substantial
variability exists in the published treatment protocols including
those that use catheter directed therapy without ultrasound.
Protocols have included upfront thrombolytic bolus as high as 8
mg of r-tPA, hourly catheter r-tPA doses from 0.5 mg to 1 mg, with
total r-tPA doses ranging from 12 mg to 35.1 mg [4,16-24]. While all
published reports are free of ICH, there continues to be major and
minor bleeding complications [4,16-24]. In a recent review of all
USAT studies, the rate of major bleeding complications was 3.6%,
with procedure related minor bleeding complications occurring in
10.7% [27].
In this report, a non-bolus, fixed, low dose, USAT protocol is
utilized in the treatment of high Intermediate Risk PE. This protocol
proved to be highly effective in reducing clot burden and acute
pulmonary hypertension without bleeding related complications.
We hypothesize that our excellent safety profile is related to the
lack of upfront r-tPA bolus, the low dose hourly infusion rate of
r-tPA, and the utilization of low dose IV unfractionated heparin for
which an aPTT of 40-60 seconds is targeted during USAT. The inhospital
mortality rate for this cohort was 1.9% (n=1/52). The one
death was attributed to recurrent PE with paradoxical embolism
through a documented Patent Foramen Ovale (PFO). The embolism
unfortunately resulted in a catastrophic cerebral vascular accident.
A PFO in PE is associated with an increased risk of complications
[28]. During this period of study, we have incorporated several
valuable components in our USAT protocol. First, all USAT
candidates undergo transthoracic echocardiography with agitated
saline to evaluate for clot within the right heart chambers and PFO.
Secondly, all USAT candidates undergo bilateral lower extremity
ultrasound for evaluation of proximal DVT.
Thirdly, at the time of catheter insertion, IVC venography is
performed to evaluate for vena cava thrombus. Lastly, if large
proximal lower extremity DVT is identified, retrievable IVC filter
is considered. While much of the focus of USAT is rapid reversal
of RV dysfunction, the long-term impact of USAT on RV function
and development of a post PE syndrome or CTEPH is unknown.
The development of CTEPH following an acute PE is believed to be
uncommon, occurring at a rate of 0.5-4% [29]. However, CTEPH
may occur more frequently following a Intermediate Risk or
High Risk PE [30]. Risk factors for CTEPH include large acute clot
burden, systolic pulmonary artery pressure greater than 50 mmHg
at the time of diagnosis of acute embolism, and systolic pulmonary
artery pressure greater than 50 mm Hg at the time of hospital
discharge [8-14,30,31]. Recent studies have suggested that USAT in
Intermediate Risk PE may reduce persistent RVD and pulmonary
hypertension when compared to standard anticoagulation therapy
alone [9,18]. Thirty-eight in this cohort had PASP > 50 mmHg by
initial catheterization, 31 of which had persistent elevation in PASP
> 50 mmHg at the conclusion of USAT.
Persistent pulmonary hypertension following USAT may be
a marker of pre-existing pulmonary hypertension or a risk factor
for the development of CTEPH. One advantage of our protocol is
that we were able to directly assess clot burden reduction, but this
did not correlate with pulmonary pressure reductions. The 55%
reduction in Miller Index is similar to reported reductions from
systemic thrombolysis [25]. The lack of correlation between clot
reduction and Pulmonary pressures may have occurred because we
did not account for cardiac output improvement or calculate PVR.
Other possibilities to explain this discrepancy includes an element
of acute pulmonary vasoconstriction, already the establishment of
CTEPH or other causes of pre-existing pulmonary hypertension.
The main limitations of this study include the retrospective single
arm analysis with small sample size. Although this standardized
protocol demonstrated safety and efficacy, the optimal infusion
rate, total dose of thrombolytic and duration of USAT remains
unanswered.
A randomized trial designed to determine the optimal dose
of thrombolytic and duration of the ultrasound procedure as
a treatment for Intermediate Risk PE (OPTALSE PE) has been
published [32,33]. Our experience with a lower infusion dose of
TPA gives further support to the safety of the procedure. Lastly,
this study lacks long-term follow up, and therefore, conclusions on
the impact of USAT on the development or prevention of the post
PE syndrome or CTEPH cannot be drawn. Our selection criteria
of utilizing USAT on cases that presented with elevated RVSP
may have biased our results with a group of patients that already
have pulmonary hypertension explaining the limited reduction
in PAP at 24 hrs compared to prior trials [21,23]. In conclusion,
this study adds to the growing body of evidence that USAT with
concurrent low dose heparin is safe and effective in reducing acute
pulmonary hypertension and clot burden in high Intermediate Risk
PE. This protocol incorporates additional considerations for the
use of USAT in Intermediate Risk PE; specifically, lower extremity
ultrasonography, initial RVSP>50 mm Hg, IVC venography, and
contrast echocardiography. These concepts should be considered
for future study design protocols. Further studies are required to
define the role of USAT in the treatment of High Intermediate Risk
PE and the impact on the post PE syndrome and CTEPH.
For more Articles on : https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.