The Application of Nanobody in Non-invasive Imaging of Cancers
Introduction
Imaging is a useful and essential tool for making the correct clinical decisions for many diseases, including cancer. Many different imaging modalities have been developed ranging from conventional microscopy methods, aimed at single cells and multiphoton intravital microscopy, to non-invasive methods at the organismal level, such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging, computed tomography (CT), bioluminescence and ultrasound imaging. The ability to image biological processes of a living animal and to diagnose signs of disease, have always been desirable goals. With the ongoing development of targeted therapies, it has become more and more important to visualize the presence tumor antigens and immune infiltrates to predict responsiveness [1].
There are several factors to be necessarily considered for designing a specific imaging agent. Once a tracer is injected into the blood stream, it must penetrate the tissue and then bind to its target. A tracer may accumulate in a tissue without binding specifically to its target. Furthermore, immunohistochemistry is needed to perform to confirm specificity and characterize the sensitivity of a tracer. Molecular imaging with labeled antibodies, extensively with labeled monoclonal Abs (mAbs), has been intensely explored, due to their particular characteristics such as high affinity and high specificity, and considered one of the best biomolecules applied for detection and targeting purposes. This can be useful for research, diagnostics, and therapeutic applications [2]. The application of antibodies in molecular imaging can help to overcome the challenge of specificity. Antibodies exist for many cell-surfaceavailable markers. Antibodies can detect cancer-specific markers and identify components of the tumor Extracellular Matrix (ECM) or tumor-infiltrating immune cells. Using radiolabeled antibodies and antibody fragments as imaging agents can be able to visualize and track location, movement and quantity of the target molecule, thereby showing insight into its dynamics.
However, antibodies’ difficult tissue penetration and longer serum half-life are strong obstacles in creating high-contrast images and cancer detection. The optimal non-invasive imaging agent would be able to penetrate tissues to allow rapid imaging after injection and show high specificity and sensitivity. The patient’s radiation exposure time should be minimized. Single domain Abs or commonly named nanobodies (Nbs), produced mainly in camelids such as llamas, alpacas, or camels, are only 15- kDa small size and improve the penetrability when compared with the performance of conventional mAbs (150 kDa) [3]. Moreover, Nbs own the characteristic of rapid renal clearance, avoiding toxicity effects [4]. One of the main advantages of obtaining Nbs by recombinant technology is that several tags can be fused in their tertiary structure such as His-tag or even fluorescent labels like the green fluorescent protein (GFP) [5]. Considering these characteristics, Nbs are particularly suited for targeting tumors and non-invasive imaging. Thus, Nbs form quite suitable candidates, ensuring minimal non-target retention to create a high tumor-tobackground ratio (T/B) shortly after administration.
Nanobody
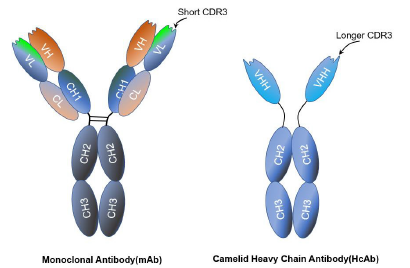
Nbs, the single domain antigen-binding fragments obtained mainly from the Camellidae such as llamas, alpacas, or camels. Normally, IgGs are formed from four polypeptidechains comprising two light chains (L) and two heavy chains (H). These host animals have the ability to produce immunoglobulins which only contain the heavy chain (HcAb) and completely lack the light chain. The heavy chain is structured into two constant regions (CH2 and CH3), a long hinge region, and the Ag-binding domain VHH [6]. Specifically, VHH is formed from different regions, ones that are more conserved (FR) and others that are responsible for the specific recognition of the Ag, called complementary determining regions (CDRs) [7]. Nbs present three CDRs instead of six occurring in conventional Abs [8,9]. The one called CDR3, usually longer than the VH domains of mAbs, being the region that shows best degree of recognition [4] (Figure1). Nbs have numerous attractive advantages over conventional monoclonal antibodies (mAbs) [5-7] include small size (15 kDa), high stability, high solubility and specificity, ease of genetic design and excellent tissue penetration in vivo.
The folding of CDR3 loop and the hydrophilic content of the framework-2 region keeps Nbs high solubility in aqueous solutions and lack of aggregation [10]. High thermal stability keeps Nbs full binding capacities for 1 week at 37℃ [11], and even completely reversible after long incubation periods at 90°C [12]. High tolerance against extreme pHs makes Nanobody great stability between pH 7.4 and [10,13] as well as in the presence of proteases [14]. The optimal biophysical and biochemical properties allow Nbs to be used for diagnostic purposes.
Recognition of Hidden Epitopes
Crystallographic studies of Nbs have revealed that in most cases the Ag-binding surface is clefts and cavities [15]. The lack of variable light chain (VL) is balanced with a VHH region that shows an extended CDR1 and a more exposed CDR3. These structural changes allow Nbs to bind planar surfaces and cavities, and also possibly bind the protruding loops or clefts [16]. Therefore, this feature of Nbs and their smaller size explain the ability of Nanobody to bind and neutralize targets that are notoriously difficult to hit with conventional Abs.
The Development and Production of Nbs
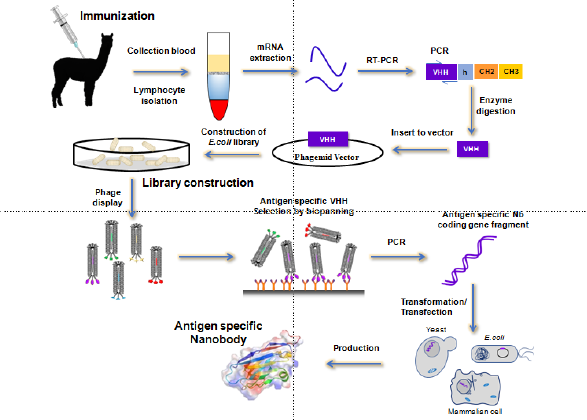
Obtaining libraries that contain the required genetic information is critical to produce Nbs with high specificity and affinity properties. At present, there are mainly three technologies for Agspecific Nbs’ preparation including immune, naïve, or synthetic libraries [9]. Immune libraries are the most common option for the development of Nbs, which requires an active immunization of Camelidae animals. Once the specific sequence is amplified from the extraction of mRNA from isolated lymphocytes and inserted in a cloning vector, the screening process is performed to isolate the most suitable Nbs by taking advantage of phage display technology, or using other methods like cell surface display and so on (Figure 2) [9]. However, phage display selection is the most commonly used strategy for this sort of screening, which is relatively fast to produce Nbs and has low cost, compared with the conventional polyclonal and monoclonal Abs.Nanobody selection based on naïve libraries takes advantage of the natural immunological diversity of the host animal without immunization [3,17]. Clearly, in any case, the success of this process depends on the amount of blood samples collected and it should be taken into account that only high specificity Abs can be obtained.
Other strategies from semisynthetic/ synthetic libraries are based mainly on randomly varying the corresponding CDR sequences to generate higher degree of diversity than when the protocol performed depends on naïve libraries. Therefore, these sorts of libraries are considered a promising alternative to the conventional method including immunization of animals. Regarding of Nanobody production, a wide range of different expression models can be used including organisms such as bacteria, yeast, fungi, insect cells, mammalian cells, or even plant hosts [18]. The most widely used expression system is Escherichia coli, which expresses proteins in different cellular compartments. The main advantage of working with this expression host is that it enables the production of soluble functional Nbs and that requires cheap protocols. Conversely, the yields are not very high compared with organisms such as yeast or fungi. Another usual way to produce Nbs uses mammalian cells.
This is the most suitable choice when Nbs are produced for therapeutic purposes, although their cost, long time requirements, and complex handling do not make them the first option. Other possible methods include the use of yeast and fungi, which have already been successfully applied, but the production process is still complex. Moreover, the fact that Nbs can be expressed in different organisms is an advantage with respect to conventional mAb production since it allows insertion of customized tags, production at low cost, and high production scale [2]. Unlike the mAbs production, which requires sophisticated machinery only found in eukaryotic systems and uses very large mammalian cell cultures and long screening and purification steps, leading to very expensive production costs, Nbs are a good alternative to solve the problem of mAb production costs. Nbs can be easily expressed in microbial systems such as bacteria, yeasts, fungi 9 and rapidly screening from display libraries. Moreover, using sequencing technologies, it is particularly easier for high-throughput screenings. All of these production and selection advantages result in lower manufacturing prices.
Introduction to Molecular Imaging Technologies
The focus on the diagnosis of tumor imaging is just critical, as the tumor’s antigen profiles obtained by visual imaging are essential to maximize therapeutic efficacy. A variety of imaging modalities are utilized in cancer diagnosis, and molecular imaging techniques have shown potential in improving existing techniques [1]. Mainly, there are two imaging techniques mentioned frequently, including nuclear imaging technique and the optical imaging technique. The nuclear techniques of PET and SPECT comprise the majority of molecular imaging studies due to the advantages of their high sensitivity, quantitative output, and clinical relevance. For tracking, Nbs are tagged with a positron-emitting nuclide (e.g., 18F, 68Ga, 89Zr) for PET, and gamma-emitting nuclides (e.g., 99mTc) are used for SPECT [1]. The optical imaging techniques, including ultrasound, quantum dots, and magnetic resonance imaging (MRI), have also been studied with Nbs. Nbs tagged with fluorescent dyes, offers the advantages of simplicity, flexibility, cost effectiveness, and safety, although the technique has weaker penetration.
Ultrasound imaging utilizes reflected sound waves from tissues, and Nbs have been tagged to contrast agents, microbubbles, and nanobubbles. Even though it is a comparatively safer technique, its applications are currently limited to systemic vasculature [19]. Quantum dots are fluorescent nanocrystals that have recently demonstrated tumor imaging potential for their superior stability, adaptable properties, and multiplex detection. However their low biocompatibility limited their current implementation. Nanobodyconjugated quantum dots targeting epidermal growth factor receptor vIII (EGFRvIII) [20], carcinoembryonic antigen (CEA) [21], and cytotoxic T lymphocyteantigen-4 (CTLA-4) 22 have achieved enhanced targeting with minimal toxicity in vivo [20,22]. MRI is a more expensive technique that utilizes strong magnetic fields to generate higher resolution images. Nbs coated magnetoliposomes [23], super paramagnetic nanoparticles [24], and fluorescent streptavidin [25] has paired with the technology for detecting ovarian tumors.
Imaging Cancer Biomarkers Against by Nbs
Currently, Nbs against cancer biomarkers, such as human epidermal growth factor receptor type 2 (HER2) are in clinical testing [26-28]. HER2, an oncogene that encodes a transmembrane tyrosine kinase receptor, is used as a classifier of invasive breast cancer and a major therapeutic target. HER2 is over expressed in 15-20% of patients with breast cancer [29-30]. Based on the success of a phase I clinical trial of a 68Ga-HER2 nanobody that could detect primary and metastatic tumors without adverse effects [31], the phase II clinical trial was performed. Notably, the HER2-CAIX combination synergistically enhanced the T/B ratio and could also detect lung metastases [32]. Nbs targeting other cancer biomarkers, such as a sepidermal growth factor receptor hepatocyte growth factor [33], carcinoembryonic antigen [34] and HER [35]. have been developed, radiolabeled and used in mouse models. Notably, vascular cell adhesion molecule-1 (VCAM-1) is a marker associated with metastasis and immune evasion, and anti- VCAM-1 nanobody microbubbles have been used for ultrasound imaging of murine carcinomas [19].
Additionally, 89Zr-HER3 [35], 18F-HER2 [36], and 68Ga-NOTACD20 [37] Nbs, 99mTc-EGFR [38] 99mTc-EGFR-cartilage oligomeric matrix protein (COMP) [39], 99mTc-dipeptidyl-peptidase-like protein 6 (DPP6) [40], 99mTc-mesothelin [41], and 131I-HER2 [42] nanobody probes have also demonstrated high T/B ratios. Additionally, anti-EGFR nanobody probes have been utilized in dual-isotope SPECT [43] and optical imaging 44, with an enhanced T/B ratio vs. mAb-based probes [44,45]. Other studies have assessed nanobody probes targeting immune checkpoints (ICP) CTLA-4 and programmed death ligand 1 (PDL1) [45] for nuclear imaging with high T/B ratios [46,47] have demonstrated success in various tumor models. An anti-human PD-L1 nanobody was developed for non-invasively imaging [48], which can detect PD-L1 in melanoma and breast tumors and showed high signal-to-noise ratios in tumors. Compared with immunohistochemistry, Wholebody noninvasive imaging of PD-L1, is likely to be more informative, which can provide visualization, localization and quantification of its expression throughout the body.
The studies published recently about a 99mTc-labeled anti- PD-L1 nanobody at an early phase I, showed that no drug-related adverse events were observed. Tumor images with good signalto- background ratios were obtained 2 h post injection and signal was mainly detected in the kidneys, spleen, liver and bone marrow [49]. Overall, Nbs have proved to be excellent imaging agents to assess the presence or absence of important cancer biomarkers on metastatic lesions and primary tumors according to the results shown from several preclinical [37,50,51] and early clinical imaging studies [31,52].
Outlook
While we have focused mainly on image a range of infectious diseases, Nbs, possessing the own advantageous physicochemical properties, such as the high tolerance of Nbs against extreme pHs, high temperatures and high concentrations of organic solvents have opened a wide range of applications for the detection of small molecule. Their nano-size enables enhanced tumor penetration and access to hidden and/or intracellular epitopes, their stability and manufacturing ease are favorable for large-scale production, and their superior paratope diversity allows an extensive arsenal for tumor antigen targeting. Nbs owning high sequence similarity with human VH domains 52 possess low immunogenicity and are appropriate for human administration. Combined with their size, structure, low agglutination, coupling efficiency, tissue penetrability and rapid renal clearance and no side effects, Nbs are a real desirable for imaging purposes. Nbs can overcome some of the limitations that first-generation Abs showed.
Using nanobody-based imaging probes has shown improved visualization compared to traditional mAb-based probes. For high affinity Nbs’ development, considering about the animal welfare, semisynthetic/synthetic libraries have been used for producing high affinity Nbs instead of the immune antibody library. However, there are still more requirement of rational and faster panning methods are applied to ensure the production of Nbs with the feature of high affinity and selectivity. With several Nbs having advanced to the clinic, and with FDA approval of one nanobodybased drug, in addition to imaging applications of Nbs, we forecast that, Nbs will be the leading actor to being developed as many innovative and high potential molecules for cancer immuneimaging and immunotherapy in the near future.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.