Recent Trends in Analytical Techniques for Impurity Profiling
Perspective
Quality and purity parameters remain under the spotlight while focusing on the safety of the drug product. The imp urities have been defined by the International Conference on Harmonization (ICH) guidelines to be the component of a new drug product, which is not the drug substance, nor the excipient added in the formulation. However, no drug substance and product is 100% pure, if one looks into depth for the analysis of impurities in the product. Considering this, guidelines have established the limits of impurity identification according to the daily dose of the drug products [1]. Three types of impurities are known and are classified by ICH as; organic, inorganic, and residual solvent impurity based on the nature and source of origin. Toxicity of impurities is the reason behind the continuing approach to detect and control them in pharmaceutical drug products. Interestingly, a smattering of impurities does not pose health risks while some have the potential to cause significant damage to human health including physiological damage, organ- and genotoxicity [2]. Consequential toxicities of impurities have been continuously investigated and reported in the active pharmaceutical ingredient (API) and synthesis materials of numerous drugs including pantoprazole [3], ceritinib [4], ranitidine [5], metformin [6], atorvastatin [7] and many more. Impurity profiling is the principal step towards controlling impurities in pharmaceuticals. The process of identification refers to) and qualification (acquiring and evaluating biosafety data) of impurities are the two main components ascertained during impurity profiling.
Outstanding advances have been observed in the development of analytical instruments for impurity profiling of pharmaceuticals. A brief overview of recently emerged and increasingly used techniques are covered in this editorial. Mass spectrometry (MS) has found significantly increasing applications in the analytical field including analysis of impurities, proteomics, pollutants, and polymers. The different forms of MS including inductively-coupled plasma MS (ICP-MS), ultra-performance liquid chromatography – MS (UPLC-MS), liquid chromatography-quadrupole time-offlight high-resolution MS (LC-Q-TOF-HRMS), vacuum outlet gas chromatography MS (GC-MS), Fourier transform ion cyclotron resonance MS (FT-ICR-MS), and other sophisticated techniques were used in impurity profiling purposes. Drugs including alfentanil hydrochloride [8], arginine vasopressin [9], difluprednate [10], cefteram pivoxil [11], alalevonadifloxacin [12] and many other have been recently profiled for impurities by MS techniques. On the other hand, electrophoretic and spectrometric techniques have also been brought into use for impurity analysis. [13] has employed nuclear magnetic resonance (NMR) for the impurity analysis in rat urine and feces. Interestingly, analytical methods for impurity profiling have also been developed using capillary electrophoresis. Impurity profiling of drug products containing biomolecules, drugs with stereochemical centers, and biopharmaceuticals can be performed using capillary electrophoresis [14].
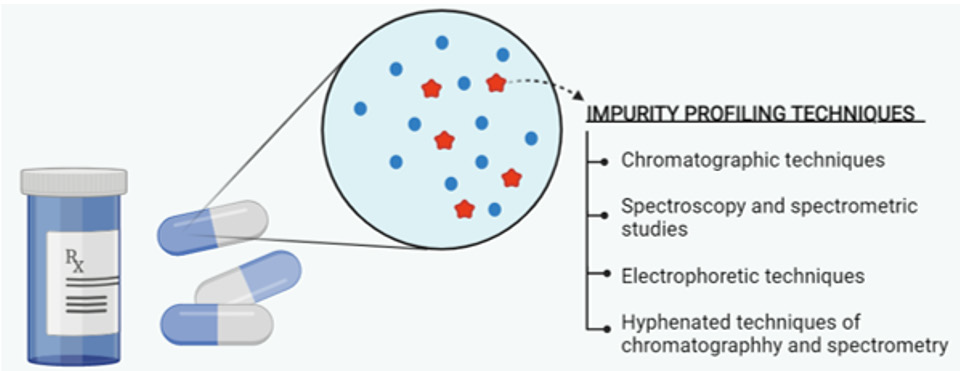
The technologies employed in impurity profiling are depicted in (Figure 1). Besides, maturing updates in chromatographic separation have been developed to efficiently execute a broad range of functions. Chromatographic techniques including hightemperature liquid chromatography (HTLC), hydrophilic interaction liquid chromatography (HILIC), supercritical fluid chromatography (SFC), UPLC, GC, and size exclusion chromatography (SEC) have established their scope of utilization in impurity profiling of drug products. Moreover, the hyphenation of chromatography with spectrometric techniques is also practiced. The equipment update in chromatographies for column and detector systems has also been observed. The control of impurities in drug products can be done if analysed. The methods of analysis have got significant advancements in recent years. The chromatographic and spectrometric systems are continuously getting new updates and being merged to expand their scope of applications. Observing at a large scale, the hyphenation of chromatographic and spectrometric methods is mostly used for impurity profiling. However, specific analytical methods for impurity profiling of drugs need to be developed.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.