Pathogenic Microorganisms in Meat Products
Introduction
Ready to eat meat products are highly demanded due to their high biological value, reasonable price, agreeable taste and easily serving. Meat products are considered as excellent sources of high quality protein, minerals and vitamins [1,2]. In Egypt, the meat products such as sausage, beef burger and luncheon are gaining popularity because they represent quick easily prepared meat meals and solve the problem of fresh and expensive meat shortage, which is not within the income reach of many families with limited income [3]. Food borne diseases caused by E. coli, Salmonella species and Staph. aureus that transmitted mainly through consumption of contaminated food and the presence of them in meat and raw meat products has relevant public health implications [4]. Microbiological aspects are a useful way to determine the safety and quality of meat product and they may be contaminated during processing from the hands, workers clothes, knives, the hide, the gut or from the environment and transportation resulting in an inferior or even unfit quality for human consumption.
The most important bacterial pathogens in beef and meat products that are responsible for food borne infection includes E.coli , salmonella and coagulase positive s.aureus [5,6]. The bacterial contamination and hygienic measures during meat production and bad storage conditions for frozen meat products can be measured using the aerobic plate count, total psychrotroph counts ,Total Enterobacteriaceae, total Coliforms and Escherichia coli biotype 1, which is the most important indicator for faecal contamination [6,7] As the contamination of meat products such as lanchoun, beef burger, kofta and Frankforter with different food-borne pathogens constitutes dangerous problems for consumers. So, this study was done to evaluate the pathogenic microorganisms in the examined meat products (lanchoun, beef burger, kofta and Frankforter) which were collected from different markets in Elgharbia governorate.
Material and Methods
Collection of samples
A total of 100 random samples of meat products (100 gm of each sample), were collected from different localities (Tanta city ,centers and villages) at Elgharbia governate. Each sample was kept in a separate sterile plastic bag and put in an ice box then transferred to the laboratory under possible aseptic conditions without undue delay and examined bacteriologically to evaluate the bacterial quality and the hygienic health hazard of them with some food borne pathogens.
Preparation of the Sample
Twenty five grams of frozen meat samples (kofta, farnkfort beef, lanshon and beef burger) under examinations were taken under aseptic condition to sterile stomachers bag then add 225 ml sterile 0.1% peptone water , the contents were homogenized at stomacher (MA 106402,FRANCE,450 to 640 strokes per minute) for 2 minutes, the mixture was allowed to stand for 5 minutes at room temperature .the contents were transferred into sterile flask and thoroughly mixed by shaking and 1ml was transferred into separate tube each containing 9 ml sterile 0.1%peptone water , from which tenth - fold serial dilutions were prepared.
Bacteriological Examination
Determination of Total Aerobic Plate Count [8]: A 0.1 ml from each of chosen prepared dilution was inoculated separately onto duplicate sterile plates of plate count agar. Spread the inoculum using sterile bent glass, the plates were incubated 35℃ for 48 hours. Plates containing 25-250 colonies were countered and aerobic plate count (APC)per gram of the sample was calculated and recorded.
Determination of Total Psychrotrophic Count [9]: Method applied according to (APHA,2001) [9], the inoculated plates are incubated at 4℃ for 10 days. Accordingly, the total psychotrophic bacterial count per gram was calculated on plates containing from 25 to 250 colonies.
Determanation of Total Enterobacteriacea Count [8]: The technique recommeneded by ICMSF,1996 using the surface plating method using violet red bile glucose agar medium (VRBG). The plates were incubated at 37℃ for 24 hours. All purple colonies were then counted and the total number of colonies was determined. Hence, the enterobacteriaceae count /g was calculated and recorded.
Determinaton of Total Coliform Count [8]: The same technique of the previous surface plating method was applied using violet red bile agar medium. The plates were incubated at 37℃ for 24 hours. All pink colonies measuring 0.5mm or more in diameter on uncrowded plates were then counted and the average number of colonies were determined. Multiply the number of colonies by the dilution to obtain the number of coliform organisms per gram of sample.
Isolation of E.Coli and Sereological Identification According to Edwards And Ewing(1972): One ml of each previously prepared serial dilution was inoculated into tubes containing 8 ml of EC broth and incubated at 45.5℃ for 48 hrs, after that a loopful from each positive tubes of EC broth (turbidity due to acid production and formation of gas in Durham’s tube) was streaked on Eosin Methyelene blue agar plates, then incubated at 37℃ for 24 hours. Typical colonies of E.coli appeared greenish metallic with dark purplecenter. Suspected colonies were purified and inoculated into slope nutrient agar tubes for further identification.
Isolation of salmonella Species According to Quninn et al.,2002.
Determination of the Total Staphylococcus Aureus Count: Accurately, 0.1ml from each of previously prepared serial dilutions was spread on duplicated plates of mannitol salt agar using a sterile bent glass spreader. The inoculated and control plates were incubated at 36℃ for48 hours. The developed colonies were enumrated and the total staphylococci count/g calculated.
Biochemical Identification of Positive Staphylococcus Aureus Isolates (Figures 1-5):
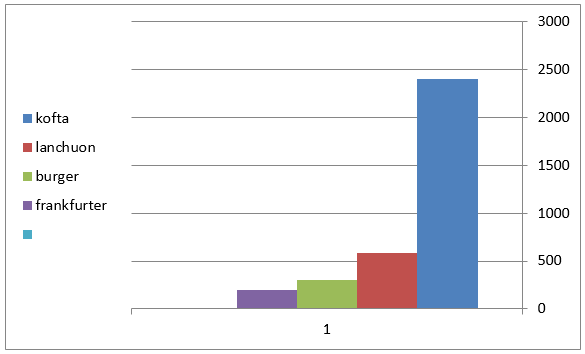
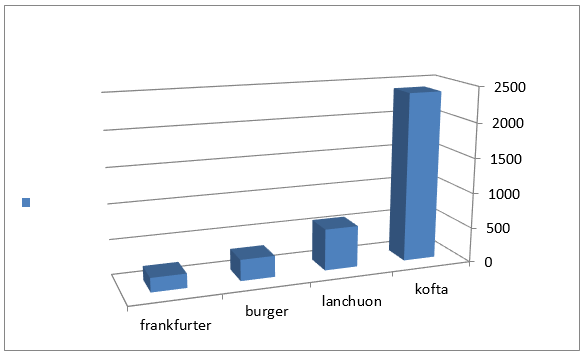
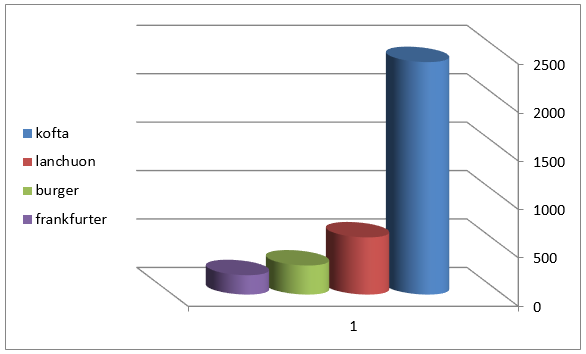
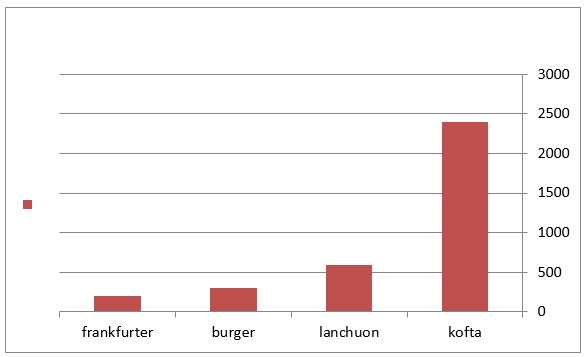
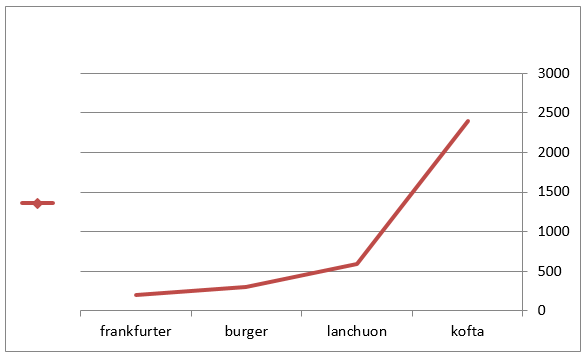
Figure 2: Mean values of staphylococcus auereus count of examined meat products(25 sample for each).
Discussion
Contamination by pathogenic microorganisms is one of the most important challenges faced by producers of processed meat products. The presence of food borne pathogens in meat and meat products can result in a range of human health problems as well as economic losses to producers due to recalls from market places. Ready-to-eat (RTE) meats are especially a concern since these may be consumed without further cooking and are known to be good growth substrates for pathogenic microorganisms [7]. The most important bacterial pathogens in beef and meat products that are responsible for food borne infection includes E.coli , salmonella and coagulase positive s.aureus [5,6]. So our study was carried out on ready to eat meat such as lanchoun, kofta, beefburgers and frankfurter at Elgarbia governorate to evaluate the bacterial count and the hygienic health hazard of them with some food pathogens.
Total Aeorobic Bacterial Count
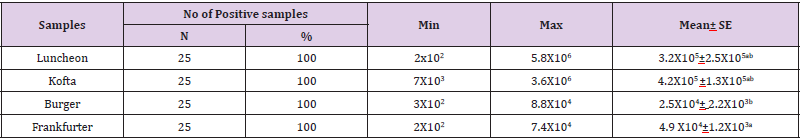
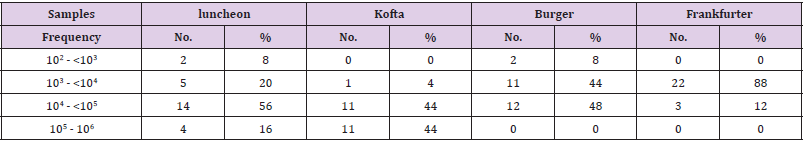
The standard plate count (SPC), also referred to as the aerobic plate count or the total viable count, is one of the most common tests applied to indicate the microbiological quality of food. The significance of SPCs, however, varies markedly according to the type of food product and the processing it has received. The data in Table 1 revealed that the minimum and maximum aerobic plate counts in the examined meat products(lanchoun, beef burger , kofta and frankfurter) collected from different localities were ranged as follow 2x102 to 5.8X106, 7X103 to3.6X106, 3X102 to 8.8X104 and 2X102 to 7.4X104 with mean value 3.2X105±2.5X105 ,4.2X105 ±1.3X105 , 2.5X104± 2.2X103 and 4.9 X104±1.2X103 respectively. All examined samples were contaminated100%. On the same side, Shaltout, et al. [10] said that the APC/g of the examined samples of ready to eat meat meals ranged from 2.1×103 to 1.7×104 with an average of 6.03×103± 1.45×103cfu/g for meat, 4.6×103to2.9×104with an average 9.91×103± 2.18×103/(cfu/g) for meat kofta (Table 2).
Table 1: Statistical analytical results of Aerobic Plate Count (cfu/g) of examined meat product samples (n=25).
Note: a-b: mean with superscripts differ significantly at p<0.05, Egyptian Standard(E.S.) (1114/2005): stated that the aerobicbacterial count should not be higher than 104
Table 2: Frequency distribution of total aerobic bacterial counts in the examined meat product samples (n=25).
Zalouk- enas(2013)examined 25 samples of ready to eat burger that collected from different localities at Giza governorate. The results revealed that , the mean value of APC were 8x 104 CFU/g that was higher than the mean value for burger in our study. Higher results were reported by [11]. Higher results may be attributed to the difference in number of the examined samples, season of work and may be there was a difference in the incubation period, as well as the high bacterial count found in the examined locally manufactured beef luncheon samples may be attributed to the contamination of the flesh meal itself that was used in the manufacture of the product, also equipment, knives and grinders were considered as a source of infection and contamination of the product during processing, Water used for washing, air, cleavers, vessels and personnel were considered another sources for contamination of the product.
Total Staphylococcus Count
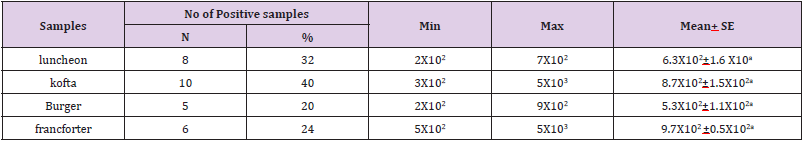
The obtained results in Table 3 cleared that the minimum and the maximum staphylococcal count in the examined samples (lanchoun, kofta , beef burger and frankfurter ) 2X102 to 7X102, 3X102 to 5X103 , 2X102 to9X102 and 5X102 to 5X103 with a mean value as follow 6.3X102±1.6 X10a, 8.7X102±1.5X102a, 5.3X102±1.1X102 a and 9.7X102 ±0.5X102a respectively. On the same side Also Shaltout, et al. [12] isolated Staph. Aureus in kofta with mean value 8.13×102 and mean value of 7.54×102 in burger. On the other hand, Staphylococcus had not been detected in beef burger or minced meat as reported by Tolba(1994), Abdel-Aziz et al. (1996),Duffy et al. (1999), Chung et al.(2003).
Table 3: Statistical analytical results of Staphylococcus aureus count (cfu/g) of examined meat product samples (n=25).
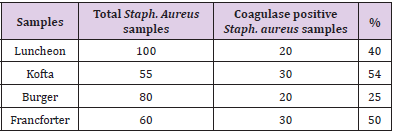
Isolation of Coagulase Positive S.Aureus
Table 4: Incidence of coagulase positive Staph. Aureus isolatd from. Different examined meat product samples.
Coagulase positive S.aureus is still major cause of food poisoning. The Table 4 revealed the Incidence of coagulase positive Staph. Aureus isolated from different examined meat product samples were as follow 20(40%) from launchon, 30(54%) from kofta, 20(25%) from burger and 30(50%) from frankfurter. This results agreed with that results obtained by Eleiewa (2003); Zaki –eman (2003); El daly et al (2014); Djoulde et al (2015)and nadimsamaa( 2016). These results disagreed with Benzerra et al (2010) and mousa, et al. [13] who isolated S aureus in higer incidence in beef burger also disagreed with Wehab and Hegazy (2007) who failed to isolate S.aureus from frozen beef burger samples. The presence of S.aureus in meat and meat products indicates poor hygiene of meat handlers as well as lack of sterilization of utensils and they grow without pronounced change in odour or taste in the products.
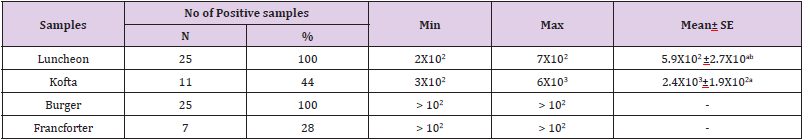
Total Psychrotrophic Count
Psychtrophic bacteria are the main cause of spoilage of meat products which are kept under refrigeration temperature due to their ability to grow at low temperature. Total Psychtrophic bacterial count can provide useful information about the keeping quality of some meat products. The results in Table 5 revealed that the minimum and the maximum of total psychrotrophic count in the examined meat samples (lanchoun, kofta , beef burger and frankfurter ) were as follow 2X102 to 7X102, 3X102 to 6X103 , > 102 to > 102 and > 102 to > 102 with mean value as follow 5.9X102 ±2.7X10ab and 2.4X103±1.9X102a respectively. As all positive samples were lower than 105, so all samples were accepted following ES(2005). these results were agree with Karaboz and dincer (2002) .
Table 5: Statistical analytical results of total psychrotrophic count (cfu/g) of examined meat product samples (n=25).
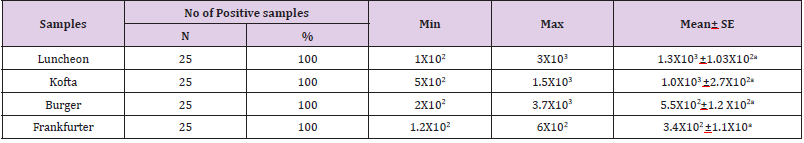
Total Enterobacteriacea Count
Table 6: Statistical analytical results of Enterobacteriaceae count (cfu/g) of examined meat product samples (n=25).
Note: The Egyptian Standard(E.S.) (1114/2005) stated that Enterobacteriaceae count and coliforms count should not be higher than 10
Enterobacteriaceae is a group of organisms are used in food testing as hygiene indicator organisms and can give advance warning of failures in hygiene procedures in food manufacturing site. Presence of Enterobacteriaceae indicates fecal contamination and microbial proliferation, which includes all the multiplication of wide range of pathogenic and toxigenic organisms and constituting a public health hazard. The public health hazard of isolated Enterobacteriaceae constituted in Escherichia coli(verocytotoxigenic) including serotype O157: H7 are one such group causing severe chronic and potentially fatal illness such as hemorrhagic colitis, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura and in severe cases death occur. The results in Table 6 revealed that the minimum and the maximum enterobacteriaceae count in the examined samples (lanchoun, kofta , beef burger and frankfurter ) were as follow 1X102 to3X103 , 5X102 to 1.5X103, 2X102 to 3.7X103 and 1.2X102 to 6X102 with mean values as follow 1.3X103 ±1.03X102a, 1.0X103 ±2.7X102a, 5.5X102±1.2 X102a and 3.4X102 ±1.1X10a respectively. Limits suggested for Enterobacteriaceae count in various foods are lower than 104 microbes /g. (center of food safety 2014). The current results were relatively agreeing to that obtained by El-Daly et al. (1987), Pivarov et al. (1988) & Elwi (1994) who found that the mean values of Enterobacteriaceae in kofta were 45 x 102/g, while higher findings were obtained by Hassan (1991), Daif (1996) & Hussein (1996) who found that the mean Enterobacteriaceae for kofta samples were 1.9 x 105/g.
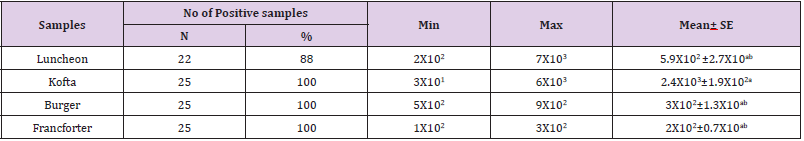
Total Coliform Count
Members of coliforms groups are referred as general indicator microorganisms to measure the potential presence of enteric pathogens in foods, besides the measuring of fecal contamination of food products and the sanitary condition in the foods processing environment. The presence of these organisms in RTE food (sandwiches) depicts a deplorable state of poor hygiene and sanitary practices employed in the processing and packaging of this food product. data in the Table 7 showed the minimum and maximum of total coliform count in the samples examined (lanchoun, kofta , beef burger and frankfurter) as follow 2X102 to 7X103, 3X101 to 6X103, 5X102to 9X102 and 1X102 to 3X102 with mean value as follow 5.9X102 ±2.7X10ab, 2.4X103±1.9X102a, 3X102±1.3X10ab and 2X102±0.7X10ab respectively . The current results agree with those recorded by El-Rayes (2008)who recorded that the mean value of total coliform count in kofta was 2.83 x 103± 0.74 x 103 /g . While, lower results were recorded by EL-Daly et al. (1987) who found that the mean values of coliform counts were 1x102 /g. In disagree with our results Stagnitta et al.(2006) examined 100 hamburgers samples to detect the microbiological and hygienic quality of meatfoods in San Luis city. They reported that the counts of coliforms in samples ranged from 101 and 103CFU/ g.
Table 7: Statistical analytical results of total coliform count (cfu/g) of examined meat product samples (n=25).
Isolation of E.coli
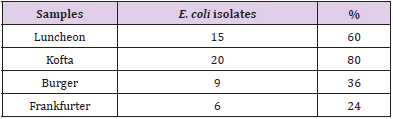
The isolation of E. coli from meat samples indicates fecal contamination and implies that other pathogens of fecal origin may be present so E.coli is considered as an indicator of fecal contamination beside , it may iduce sever diarrhea in infants and young children as well as food poisoning and gastroenteritis among adults (synge et al.,2000) Results in Tables 8 & 9 showed Incidence of E. coli isolates isolated from meat product samples (lanchoun, kofta , beef burger and frankfurter) were 15(30%), 20(40%), 9(18%) and 6(12%) respectively. Also As our results, the Escherichia coli isolated in kofta samples that reported by El-Mossalami (2003) were (40%). However, lower values were recorded by El-Taher-Omyma (1998) (25%), Saleh (2001) (16%) and Al-Mutairi (2011) 7 (28%). On the other side Mansour [14] reported that E.coli was detected in 44 beef samples and their incidence were 14 (56%), 12(48%),10(40%) and 8(32%) in kofta, burger, sausage and frankfurter respectively that was higher than our results. Also results that were found by previous investigators. Duitschaever(1977) and Fathi et al., (1992), they detected E.coli in 28.3%of the examined beef burger samples that was less than our results.
Isolation of Salmonella
In our study, Salmonella serovars were failed to be detected in all examined samples of meat products. Salmonellae were failed to be isolated from the examined samples, this may be due to thermal treatment of luncheon during processing or cooking in a temperature around 70-73℃ within it may destruct the pathogenic bacteria, also this may be due to addition of additives such as preservatives, spices and nitrites, which have an antimicrobial effect on Salmonella which inhibits their growth and multiplication. This result agreed with the legal requirement of E.S.(1114/2005). Obtained result was similar to those reported by (Amal and Seham, 1998; Hala and Hoda, 2002; Amal, 2004 and Ibrahim, 2009), all of them failed to detect Salmonellaein luncheon. While (Youssef et al., 1999 and Karmi, 2013) isolate Salmonellae from luncheon samples with an incidence 2% and 10% respectively. This may be due to highly contaminated samples, before a cooking step. On the same side, (Abdel-Aziz et al. 1996)and (Kuplul and Oral, 2003) failed to isolates salmonella from any of examined luncheon samples. Salmonella species were not found in any of the examined luncheon samples. or kofta samples).
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.