Remarks on Severity of Trauma Patients Due to Road Traffic Accidents have Been Treated at Vietduc University Hospital Assessed by RTS
Introduction
Traffic accidents are always a global problem. According to
statistics of the World Health Organization - WHO, every year around
the world, about 1.35 million people die, leading to 50 million
people being permanently disabled due to irreversible injuries,
accounting for 30-50% of total hospital admissions (3). Vietnam is
the country with the highest number of traffic accident deaths in
ASEAN and one of the countries with the most traffic accidents in
the world. Therefore, traffic accidents are always a current topical
issue in Vietnam because it’s a burden on health care and society,
affecting the patient’s lives. Although the Government has been
implementing many measures to reduce the number of cases and
victims, Vietnam is still in the group of developing countries with
high rates of morbidity and mortality caused by traffic accidents [1-4].
To improve the qualifications of trauma care, one interesting
issue is updating trends in trauma care assessment. Primary
trauma care requires assessment of severity to develop appropriate
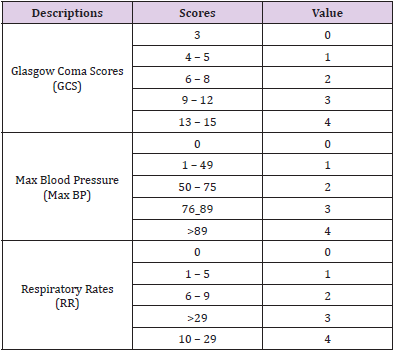
care strategies. The Revised Trauma Score -RTS simplifies the rapid
assessment of injury based on Respiratory Rate, Maximum Blood
Pressure, and traumatic brain injury severity - Glasgow Coma Scale
has been widely recognized for clinical decision making. Several
articles have evaluated the performance of RTS in the emergency
department (ED) as a triage and prediction tool and showed the
effect in clinical practice because bedside assessment tool, each
of its variables can be easily and quickly calculated [5,6]. Viet
Duc University Hospital, one of the leading centers of surgery in
Vietnam, annually receives more than 30.000 trauma patients, most
of them are serious trauma patients due to traffic accidents. Quick
triage for providing proper treatment is a very important issue for
health workers at the ED because it could impact the outcomes of
treatment. Therefore, we have conducted this study to evaluate the
effectiveness of using RTS on trauma patients at ED of Hospital.
Materials and Methods
Tool
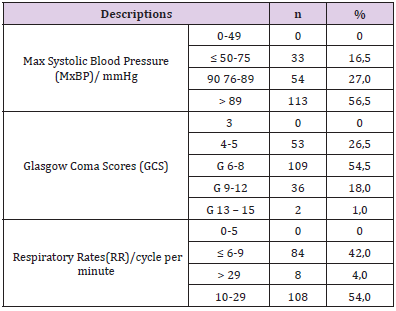
In this study, we used the RTS including three parameters are considered for the RTS: maximum systolic blood pressure (MaxSBP, mm Hg), Respiratory Rates (RR, cycles per minute), and Glasgow Coma Scale (GCS). The RTS will range from 0 to 12, where the lower RTS is the more severe injury at the higher risk of death [5] (Table 1).
Setting and Participants
We prospectively analysed the clinical data of 200 patients with traffic accident acute trauma who were treated in the ED of Viet Duc University Hospital, a comprehensive tertiary surgical hospital) from December 2020 to March 2021. The patients were assessed the RTS upon admission within the first 24 hours. The data were recorded by attending nurses and doctors at the time of the patient’s presentation to the ED. Exclusion criteria were used: The patients already had airway intervention such as endotracheal intubation, mechanical ventilation. The patients died on arrival or were discharged from the ED before termination of emergency treatment, the medical records were not completed.
Data Analysis
Data were processed using SPSS 20.0 software.
Results
A total of 200 patients who met the selection criteria were
analyzed. The characteristics of subjects are as follows:
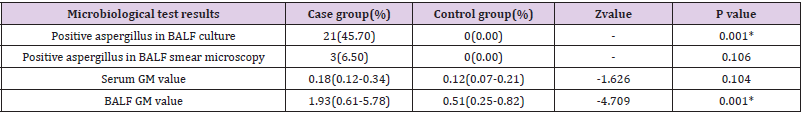
*All 50 patients who dead in the group with RTS ≤ 9. The
difference between survival and death rates of groups with RTS ≤ 9
and RTS ≥ 9 is statically significant with P <0.05.
Discussion
Injuries in general and traffic accidents, in particular, are
still a global problem. In most developed countries, the injury
classification system helps to provide appropriate care strategies,
reducing complications and mortality. However, in many
developing countries like Vietnam, the trauma emergency system
is still incomplete and has many challenges. According to Zhejun Yu
[4] each year, more than 400 000 people die in China from motor
vehicle accidents or industrial accidents, among which 1%-1.8%
were multiorgan/multisystem injuries. China’s regional trauma
system hasn’t yet been full-fledged, and the management of trauma
centers is facing great challenges. Therefore in all emergency rooms,
especially in cases of overcrowding and understaffed, it is critical
to rapidly screen large numbers of patients, identify the critically
ill patients promptly, assess the severity of their condition and
assign appropriate treatment priorities, and transfer them towards
or intensive care unit are very important issues while treating the
patients there [7-9].
In the past 30 years, a different trauma scoring system has been
developed, most of the scales are combined with factors related to
anatomy and physiology.
However, the scales are too complicated, with many variables,
while the emergency needs to be done as quickly as possible.
Among the commonly used scales are the Revised Trauma Score
(RTS) or the T-Revised Trauma Score (T-RTS), the Severity Scale.
Injury - Injury Severity Score (ISS) and Trauma Score-Injury
Severity Scores (TRISS), the RTS is widely used. Many studies have
evaluated the effectiveness of applying RTS to serve trauma care at the
ED effectively [10-12]. In 1989 Champion HR [5] has introduced
a revised scale to assess the severity of trauma based on three main
indicators: Respiratory Rates - Maximum Blood Pressure – Glasgow
Coma Scores abbreviated as RTS - Revised Trauma Score. According
to the rating scale, the lower the RTS, the higher the risk of death.
Because RTS reflects trauma severity, it is considered a useful
tool to predict the patient’s survival and death. The study of R.A
Lichtveld [13] of 503 trauma patients showed that when compared
with non-ventilated patients with unchanged RTS, the risk of death
in patients with RTS scores was 3.1 times lower (P=0.001), patients
with a good initial RTS score but subsequently intubated were 2.9
times higher (P<0.001) and in patients with a low RTS, intubated
were twice as likely (P<0.001) (6). According to Nguyen Huu Tu
[14] if RTS ≤9 mortality rate is 78.3% compared to 3.4% of the
RTS group >9 (3). Research results of Nguyen Huu Tu and Nguyen
Truong Giang are similar: the higher RTS means the greater rate
of survival [14,15]. In the study on the effects of T-RTS by Lam Vo
Hung [6] to triage of trauma patients at the ED of An Giang hospital
in the South of Vietnam in 2012 through 150 trauma patients with
traffic accidents. The study has shown that RTS had a statistically
significant difference in the mean value of the survival group with
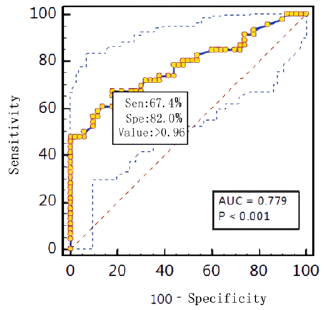
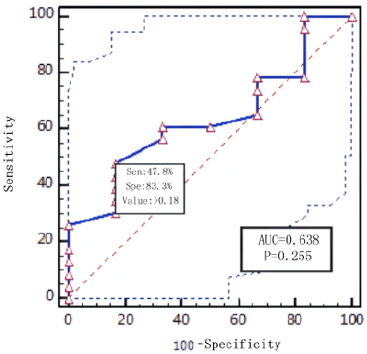
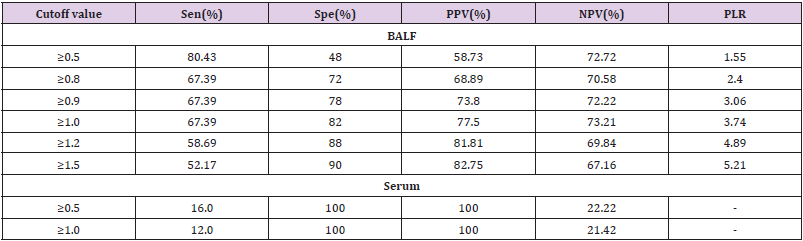
the death group with P = 0.000. RTS cut-off score <9 predicts
mortality with a sensitivity of 88% and a specificity of 99%.
The author recommends that RTS should be widely applied
in medical facilities and that the RTS scale is effective in survival
prognosis. With a sensitivity of 88.2% and a specificity of 99.2%,
the RTS shows an effective role in assessing the risk of death. The
reports of Nguyen Huu Tu, et al. [14,15] also had similar results with
sensitivity of 78.7%, 76%, and specificity of 95.1%, 84%. According
to the study by Kondo Y et al. [16] about the correlation between
long-term mortality and short-term mortality of RTS, T-RTS, TRISS,
MGAP (mechanism, GCS, age, and arterial pressure) score, and GAP
(GCS, age and arterial pressure) score. They found that T-RTS was
better at predicting short-term mortality than long-term mortality.
For the aging group, the study of Lam Vo Hung [6] showed that
the group with the highest mortality was from 16 to 39 years old,
young people who were hyperactive, disregarded traffic rules, and
easy to be injured by traffic accidents (accounting for 50% of the
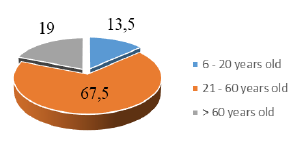
total sample of study). In our series, the age group was the highest
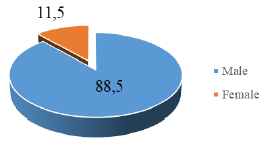
proportion from 21 to 60 years old, accounting for 64%, males
accounted for the majority of 86.7% (Figures 1 & 2). As for the type
of injury, in the study of Lam Vo Hung [6], traumatic brain injury
and multiple trauma had a high mortality rate. Among the types of
injuries, there was a statistically significant difference in mortality
with P<0.05. Nguyen Huu Tu [14] has the same comment as us, the
mortality rate due to traumatic brain injury and multiple trauma is
16.6% and 22.3%, respectively (3).
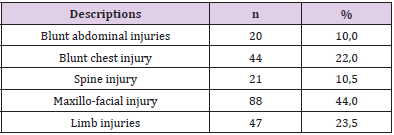
In addition, Nguyen Truong Giang [15] studied 532 accident patients at 103 Hospital and found that the RTS were low in the traumatic brain injury group and the multi-traumatic group. Nguyen Duc Chinh, et al. [17] conducted a study at Viet Duc University Hospital on deaths (2016-2018) showed that the traumatic brain injury group accounted for the highest rate, especially the group with GCS from 6 to 8. Bruno Durante, et al. [18] analyzed 200 patients from December 2013 to February 2014, including trauma victims admitted to the emergency room of the Cajuru University Hospital. The patients were set up in three groups: (G1) penetrating trauma to the abdomen and chest, (G2) blunt trauma to the abdomen and chest, and (G3) traumatic brain injury. The variables we analyzed were: gender, age, day of the week, mechanism of injury, type of transportation, RTS, hospitalization time, and mortality. Regarding mortality, there were 12%, 1.35%, and 3.95% of deaths in G1, G2, and G3, respectively. The median RTS among the deaths was 5.49, 7.84, and 1.16, respectively, for the three groups. The authors concluded that RTS was effective in predicting mortality in traumatic brain injury, however failing to predict it in patients suffering from blunt and penetrating trauma. In our study, the highest proportion of combined injuries were maxillofacial injuries 44%, limb injuries 23.5%, blunt chest injuries 22% (Table 2).
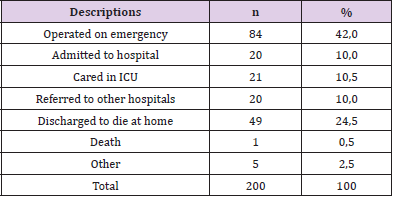
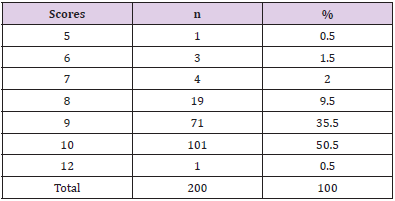
Patients with severe traumatic brain injury according to the GCS of 6 – 8 accounted for the highest rate of 52% (Table 3). Up to 40% were operated on emergency within the first 24 hours. The rate of serious injured was discharged to die at home accounted for 24.5%, 0.5 % died in hospital, overall mortality was 25% respectively (Table 4). Regarding the RTS in our study, there were mostly in the group of RST at 10 points (50.5%) and 9 points (35.5%). There were 98 patients (49%) with RTS ≤ 9 (Table 5) of which, 91.8% with serious brain injury. All 50 fatal and critically ill patients were in this group.
In a Mega-analysis of Manoochehr S [19], to compare the ability of Revised Trauma Score (RTS) and Kampala Trauma Score (KTS) in Predicting Mortality, the study was conducted by two investigations searched the Web of Science, Embase, and Medline databases and the articles in which the exact number of truepositive, true-negative, false-positive, and false-negative results could be extracted were selected. A total of 11 relevant studies (total n = 20,631) were investigated. Regarding the accuracy and performance, the author concluded that RTS was better than KTS for distinguishing between mortality and survival. Compared with the other researches of domestic and international, we find that RTS is convenient to use in a clinical emergency for trauma victims. Moreover, in addition to the GSC, RTS can also be used as a predictor of severity and mortality, helping physicians at ED to making quick decisions and providing appropriate treatment [4,6,20,21].
Conclusions
Through the study of 200 trauma patients due to traffic
accidents, we found that RTS has a value in predicting survival as
shown by the difference between survived group and the death
group. The patients who died were in the group with scores ≤ 9
statically significant with P <0.05. Because it is easy to calculate and
suitable for first aid, it is recommended to apply in clinical practice,
especially in the actual conditions of Vietnam. In the difficult
conditions of shortage of resources, the trauma emergency system
has not been standardized, the application of RTS helps to reduce
the morbidity and mortality rate.
For more Articles on : https://biomedres01.blogspot.com/